UNIT 2
POLYMER CHEMISTRY
Polymer chemists study large, complex molecules that are built up from many smaller units. They study about the smaller building blocks combine, and create useful materials with specific characteristics by manipulating the molecular structure of the monomers/polymers used, the composition of the monomer/polymer combinations, and applying chemical and processing techniques that can to a large extent, affect the properties of the final product. Polymer chemists are unique within the chemistry community because their understanding of the relationship between structure and property spans from the molecular scale to the macroscopic scale.
It is a long molecule formed by joining together of thousands of small molecular units by chemical bonds. Polymers are giant molecules also called macromolecules that are essential to our existence. They are important chemicals in our bodies in plants (starch, cellulose) and in our everyday lives (fibers, plastics, elastomers). Polymers are made by transforming small molecules into molecules with very large molecular weights. Although the chemical properties of polymers are similar to those of analogous small molecules, their physical properties are quite different. Every polymer has its own characteristics, but most polymers have the following general properties:
Polymers can be very resistant to chemicals.
Polymers can be both thermal and electrical insulators.
Generally, polymers are light in weight with varying degrees of strength.
Polymers can be processed in various ways to produce thin fibers or intricate parts.
Polymer is a molecule formed by joining of thousands of smaller molecular units together by chemical bonds. A chemical process that leads to the formation of polymer is known as polymerization. Degree of polymerization: The number of repeat units monomeric units available in the polymer is known as degree of polymerization.
Functionality: The number of bonding sites or reactive sites or functional groups present in the molecule. Ex: The double bond in vinyl monomers (CH2 = CHX) can be considered as a site for two free valencies. When the double bond is broken, two single bonds become available for combination.
H2C=CHX → CH2 – CHX
- When the functionality of monomer is two bi-functional linear (or) straight chain polymer is formed.
Ex: (a)vinyl monomers
(b)adipic acid
(c)hexamethylene diamine
(d)terephthalic acid
(e)ethylene glycol
(f)amino acid Example for polymer: HDPE (high density polythene)
2. When the functionality of monomer is three (tri-functional), three dimensional net work polymer is formed. Ex: phenol, glycerol Examples for polymers : Urea formaldehyde, phenol formaldehyde.
Monomers: Monomers are atoms that bond together to form more complex structures such as polymers. There are four main types of monomer i.e.; sugars, amino acids, fatty acids, and nucleotides. Each of these monomer types play important roles in the existence and development of life and each one can be synthesized abiotically. Monomers are commonly found in the interstellar medium, nebulae, and chondritic meteorites. The number of repeating units (n) in the chain so formed is called the Degree of polymerization (DP=n). Polymers with a high degree of polymerization are called High polymers and those with low degree of polymerization are called Oligo polymers
Functionality:The functionality of a monomer determines the final polymer that will be formed due to the combination of the monomers. The number of reactive bonds that are available for coupling will determine whether the monomer will be mono-, bi-, tri-.
Mono-functional:The presence of single reactive group in monomer molecule, then it is termed as monofunctional. However a monofunctional group cannot lead to the propagation of a polymer chain. E.g.: in carboxylic acid, CH3COOH, the –COOH group is the monofunctional group.
Bi-functional:The presence oftwo reactive groupsin the monomer molecule is termed as bifunctional. Polymerization reaction with bi functional groups occurs when a double bond splits to couple with another double bonded monomer.If a double bonded molecule is present then the polymer would be –
NR=R -(R-R)n-
Chain polymerization:
The addition polymerization is the process in which the linking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerisation and the polymer is an exact multiple of the original monomeric molecule.
Step Growth polymerization:
An intermolecular reaction involving two different bifunctional reactants with affinity for each other and taking place through repeated condensation reaction is known as condensation polymerization.
Copolymerisation:
Addition polymerisation involving a mixture of two or more suitable or compatible monomers gives a copolymer and the process is known as copolymerization. A reaction in which a mixture of two or more monomers is allowed to undergo polymerisation is known as copolymerization. The polymer is known as copolymer.
E.g.: Copolymerization of styrene and methyl methacrylate
Free Radical Polymerization: The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called as the free radical polymerization. In order to obtain the wide variety of different polymers and material composites the free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction make it one of the most versatile forms of polymerization which allows a facile reactions of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.
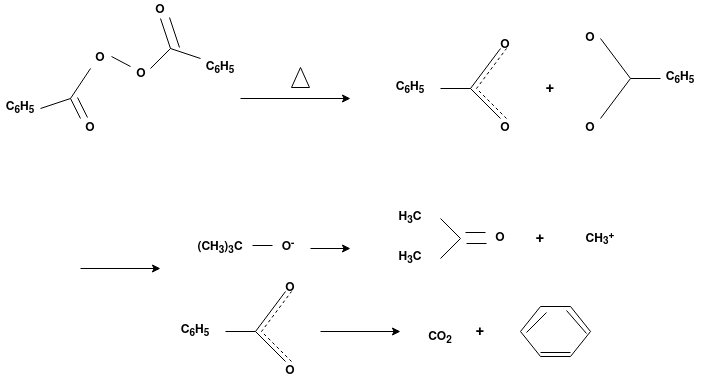
Radical Initiators
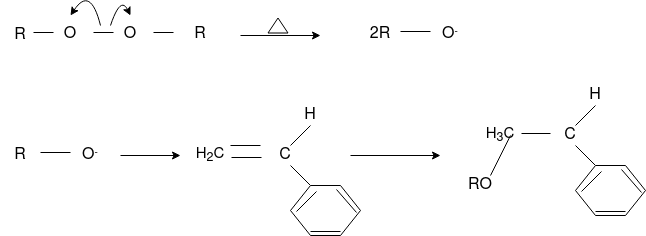
Chain Initiation
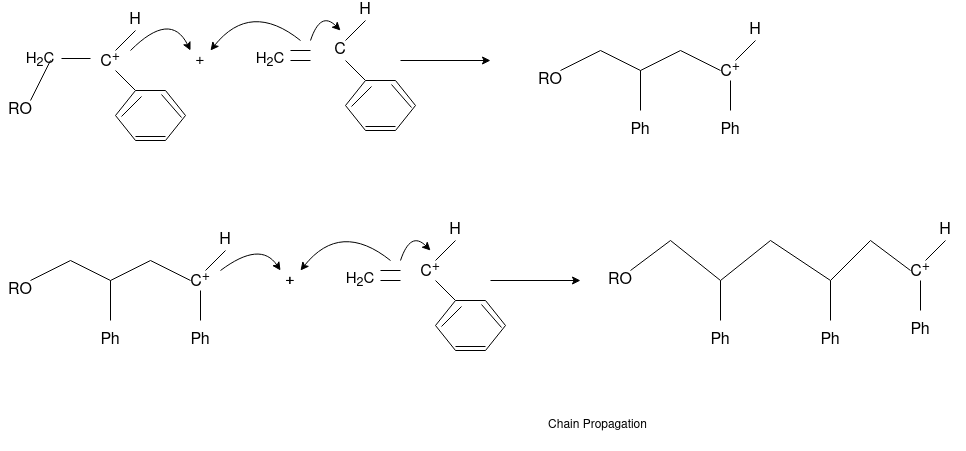
Chain Propagation
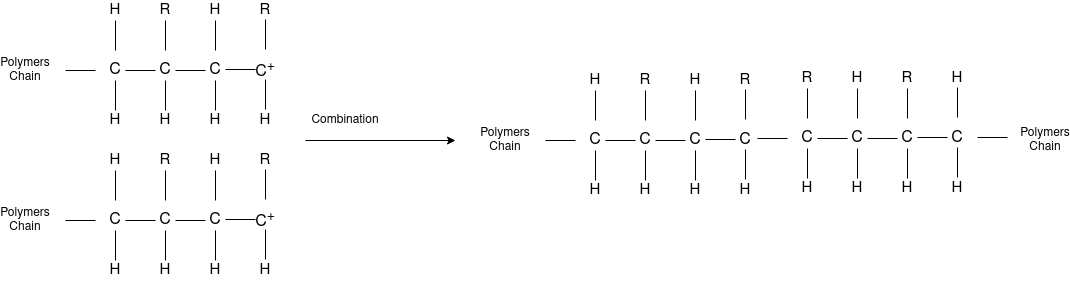
Chain Termination
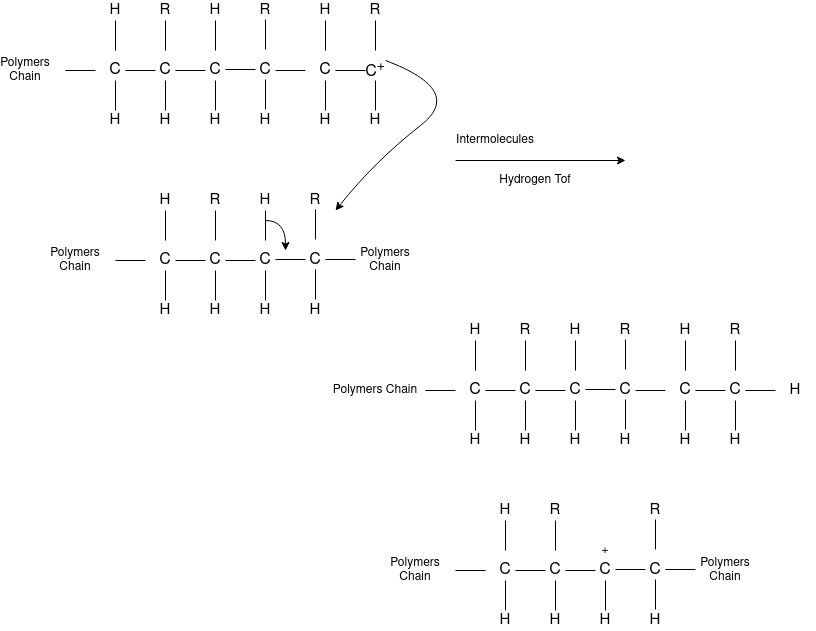
Chain Transfer Reaction
Ionic Polymerization: Alkenes can polymerize under the influence of both cationic and anionic initiators. As with free radical polymerizations, the ionic processes involve initiation, propagation and (sometimes) termination steps.
Cationic Polymerization: Cationic polymerizations are typically initiated by carbocations, generated by protonation of an alkene with a strong acid such as sulfuric or tri-fluoro methane sulfonic acid.
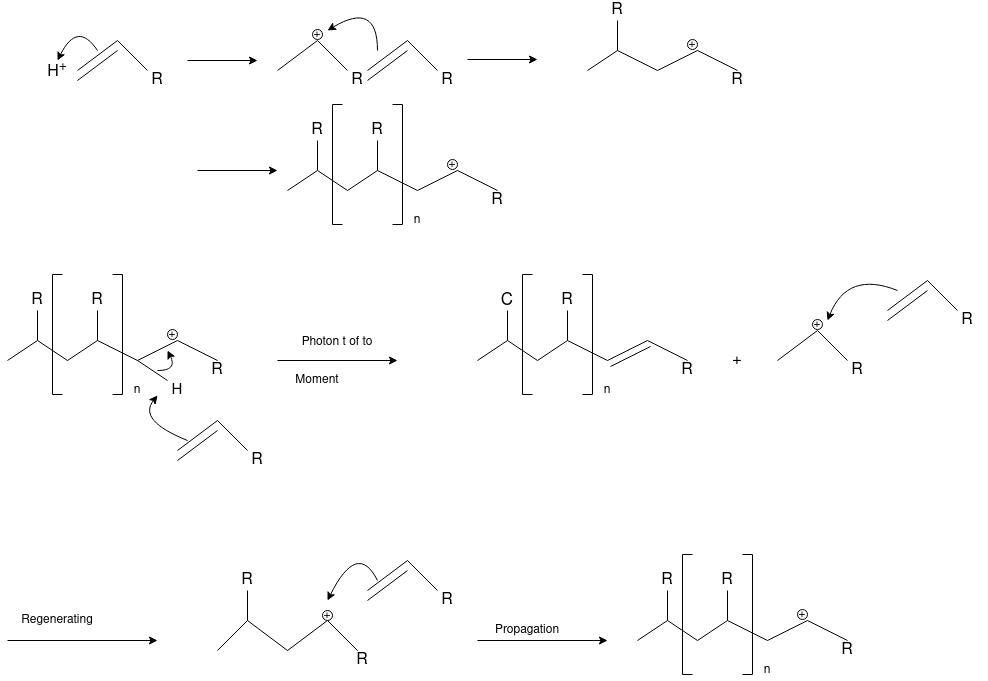
Anionic Polymerization:Anionic polymerizations are typically initiated by carbanions such as organolithium compounds.
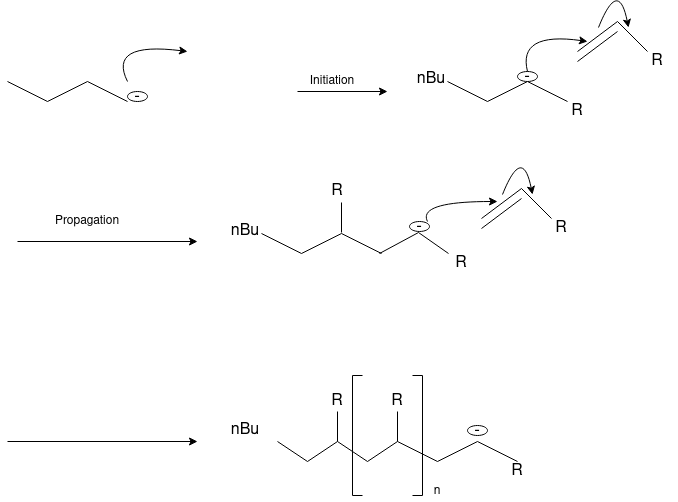
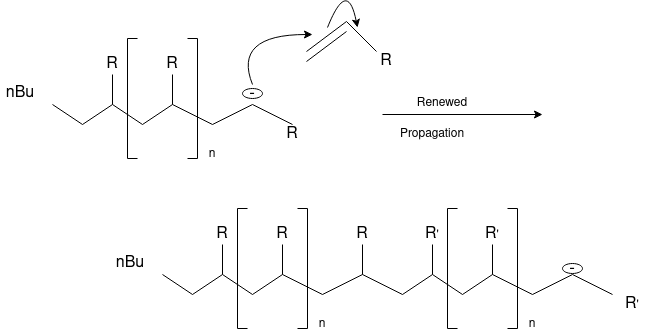
Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect its cost.
Thermoplastic polymer:A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behavior. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called crosslinks, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, a there most, as opposed to a thermoplastic, cannot return to its initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression molding, resin transfer molding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behavior. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
Preparation of Bakelite
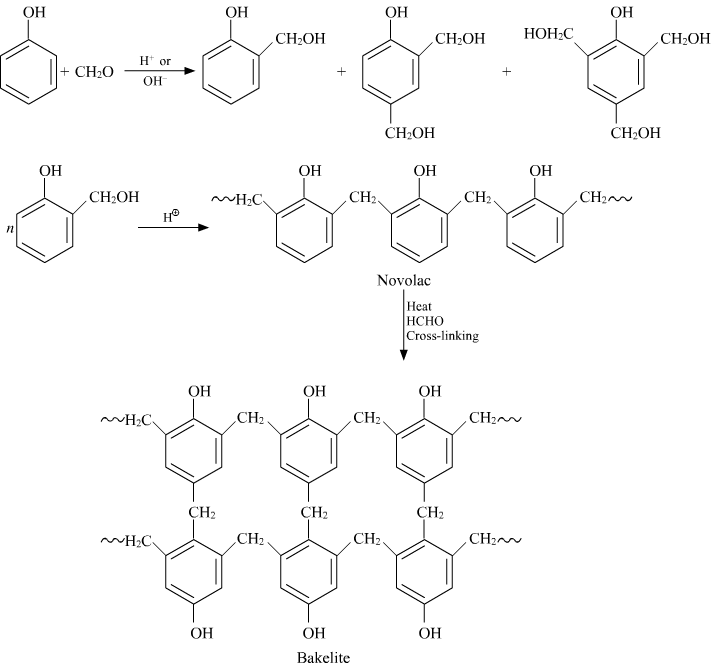
Properties: It is very smooth to mold the Bakelite. Molding is very smooth and retains any shape. They are resistant proof, scratch proof.
Applications and uses of Bakelite:
- This element has a low electrical conductivity and high heat resistance
- It can be used in manufacturing electrical switches and machine parts of electrical systems.
- It is a thermosetting polymer and Bakelite has high strength meaning
- It basically retains its form even after extensive molding.
- Phenolic resins are also extensively used as adhesives and binding agents. They are further used for protective purposes as well as in the coating industry.
Rubbers also known as elastomers, they are high polymers, which have elastic properties in excess of 300%.
Natural Rubber:- Natural Rubber is a high molecular weight hydrocarbon polymer represented by the formula (C5H8)x. It is obtained from a milk emulsion called latex by tapping the bark of the tree. “Heve a brasiliensis”. It is a polymer of isoprene units.
n H2C = C – CH = CH2 ( H2C – C = C – CH2 )n
CH3 CH3
The polymer chain of natural rubber is made of 2000 to 3000 monomer units.
Processing of Natural Rubber:- By cutting the bark of rubber tree the milky colloidal rubber milk is obtained. The main constituent of rubber latex is 25-45% of rubber and the remaining are water, protein & resinous materials. The rubber latex is coagulated by using 5% acetic acid and made in to sheets. The rubber sheets are cured under mild heat and then subjected to further processing.
Crepe rubber:- To the rubber latex a small amount of sodium bisulphate is added to bleach the colour and feed in to roller which produce 1mm or more thickness sheets which are dried in air at about 40- 500C. The dried thin sheet of rubber are known as “smoked crepe rubber”.
Mastication:- Rubber becomes soft and gummy mass when subjected to severe mechanical agitation. This process is known as mastication. Mastication followed by the addition of certain chemical (compounding) which is carried out on roll mills or internal mixers. After mastication is complete, the rubber mix is prepared for vulcanization.
Synthetic rubbers (Buna-S):
Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrine. Buna-S is the replacement of natural rubber while styrene, 2 monomers and butadiene play a major role in its derivation where as these 2 monomers is polymerized by two basically different process i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called as cold rubber. The obtained rubber is called as the Styrene Butadiene Rubber (SBR).
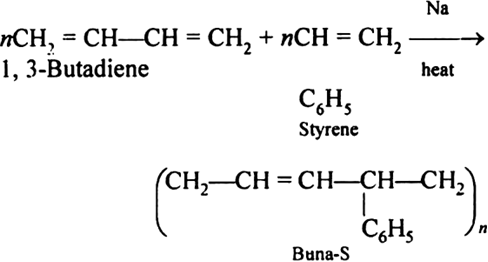
Applications:
(i) Buna-S is used as the natural rubber i.e., they are widely used in pneumatic tyres.
(ii) SBR is extensively used in coated papers.
(iii) They are highly used in building materials as a binding material.
SBR is also used as binder in lithium-ion battery electrodes.
Buna-N:
Buna-N are commonly used as elastomers. They are unsaturated synthetic co-polymer. They are obtained by the co-polymerisation of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst. Nitrile rubber, NBR or Perbunan are another terms used for the Buna-N. They are synthetic rubber copolymer of acrylonitrile (ACN) and butadiene. Elastomers is natural or synthetic material which does not break when stretched and when released it return to its original length. The most important monomer used in preparing Buna-N is Acrylonitrile rubber which gives hardness, tensile strength, fuel and oil resistance. It usually contain 34% ACN. Grinding wheels can be made with nitrile rubber. When they are used for grinding smokes or fumes are emitted, which are known as acrid. The objectionable odor can be prevented if certain classes of diketones, which contain a conjugated system of two double bonds or unsaturated groups are added. Dibenzoylethylene, chloraniland anthraquinone help to prevent odor formation.
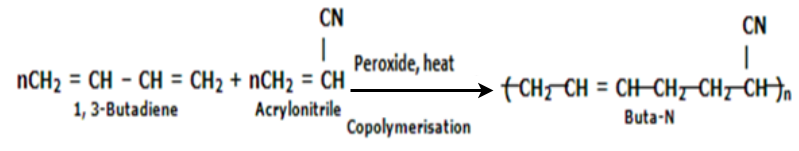
Buna-N are resistive to oil, fuel and other chemicals which means more Buna-N higher the resistance to oil but flexibility of the material is less. They are resistant to aliphatic hydrocarbons. They are non resistant to solvents but they may be attacked by ozone, ketones, esters and aldehydes.
Applications:
(i) They are used in the disposable non-latex rubbers, belts, gaskets.
(ii) They are used in preparation of adhesive and as a pigment binder.
Conducting polymers have been widely utilized in biomedical applications due to their conductivity, compatibility and low-cost process ability. In addition, compared with traditional metal or semiconductor materials, conducting polymers are more biocompatible. These advantages make conducting polymers more attractive to bioengineering researchers. The mechanism of electrical conductivity of conducting polymers is based on the transmission of polarons and bipolarons. The synthesis of conducting polymers can be accomplished by electrochemical polymerization, which is more straightforward than traditional chemical synthesis. In this chapter, several applications of conducting polymers in biomedical engineering are illustrated with examples. The applications include the use of conducting polymers in biosensors, neural electrodes, drug delivery and bioactuators. The chemical origins of such a remarkable difference in the material properties between various types of polymers can be readily -bonds and hence a charge oncerationalized. Traditional polymers, such as polyethylene or polypropylene, are made up of essentially created on any given atom on the polymer chain is not mobile. The -conjugation in polymers, however, confers the required mobility to charges that are created on the polymerpresence of an extended backbone (by the process of doping) and make them electrically conducting. One problem is that, due to the presence of this extended conjugation along the polymer backbone, the chains are rigid and possess strong inter chain interactions resulting in insoluble and infusible materials. These conjugated polymers, hence, lacked one of the most important and useful properties of polymers, namely their ease of process ability. More recently, however, it was demonstrated that when lateral substituent’s were introduced, even conjugated polymers can be made soluble (hence, process able) without significant loss in their conductivity. One other problem that plagued this field from its inception, is the inherent instability of these polymers (especially, in the doped form) to ambient conditions. Today, conducting polymers that are stable even in the doped form have been prepared. We shall highlight some specific examples of such systems and discuss some of their potential applications.
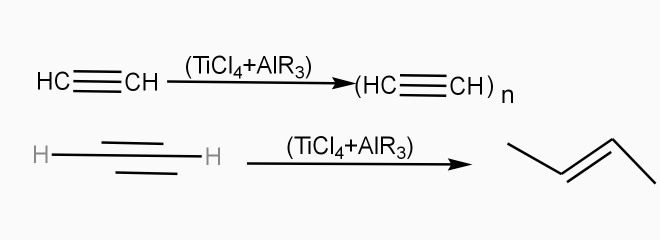
Poly acetylene: -
Structure: -
- The polymer consists of along chain carbon atoms with alternating single and double bonds between them. Each carbon possesses one hydrogen atom.it is obtained by Ziegler matter ayteslys.
- Doping of polymer: - addition of impurities
- Intrinsic polymers: - the polymer which conduct electricity on their own is called as intrinsic polymers.
- Extrinsic polymers: - the polymers which conduct electricity by doping processes are called as extinct polymers.
In doping process, polymer is either oxidized or reduced.
There are two type of doping
a] oxidative / p type doping
- Includes ICPS with Lewis acid.
- Oxidizing reagents are used (e.g. Iodine vapours)
- Oxidation taking place
- +VE change develops on polymer chain.
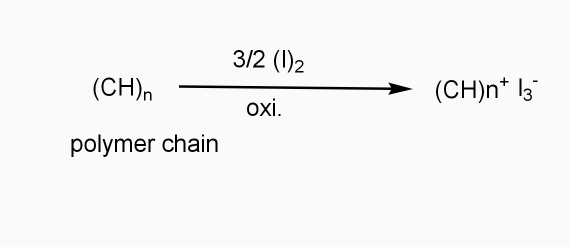
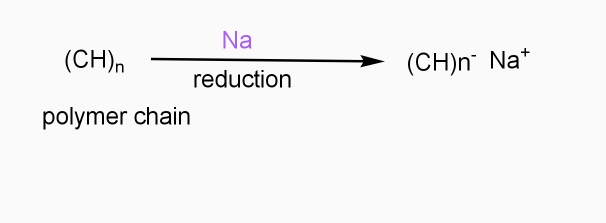
b] Reductive / n type doping
- Includes ICPS with Lewis bases
- Reducing reagents are used (e.g.Na,Li,etc.)
- Reduction taking place
- - ve charge develops on polymer chain
Applications: -
- For making rechargeable light weight batteries.
- In electronic devices, transistors, diodes.
- In optical display device.
- In solar cells.
- In telecommunication system.
The designing and making up of anything whose use depends on the specific structure at nanoscale. These posses the different properties at reducing scale. The measurement can be taken in notice at 100 nanometers and lesser is called as the nanotechnology. This includes the materials made by the manipulation of up of atoms or molecules. They are composed up of metals, ceramics, polymers, organic materials in simpler way these all are made up of carbon compounds. The technology that we deal with in taking the consideration up of the Nano material is called as the nanotechnology.
Some nano materials occur naturally, but of particular interest are engineered nanomaterials (EN), which are designed for, and already being used in many commercial products and processes. They can be found in such things as sunscreens, cosmetics, sporting goods, stain-resistant clothing, tires, electronics, as well as many other everyday items, and are used in medicine for purposes of diagnosis, imaging and drug delivery. Nano materials are found basically form following three sources:
1) Engineered
2) Incidental
3) Natural
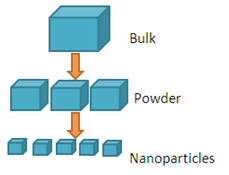
Top Down Approach
Top down approach deals with the breaking down of bulk material into nano sized particles. Top-down synthesis techniques are extension of those that have been used for producing micron sized particles. Top-down approaches are inherently simpler and depend either on removal or division of bulk material or on miniaturization of bulk fabrication processes to produce the desired structure with appropriate properties. The biggest problem with the top-down approach is the imperfection of surface structure. E.g.: nano wires made by lithography are not smooth and may contain a lot of impurities and structural defects on its surface.
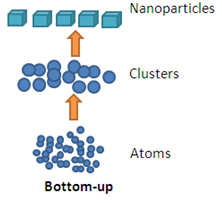
Bottom Up Approach
Bottom –up approach refers to the build-up of a material from the bottom: atom-by-atom, molecule-by-molecule or cluster-by-cluster. This route is more often used for preparing most of the nano-scale materials with the ability to generate a uniform size, shape and distribution. It effectively covers chemical synthesis and precisely controlled the reaction to inhibit further particle growth. Although the bottom-up approach is nothing new, it plays an important role in the fabrication and processing of nanostructures and nano materials.
Nano tubes: Carbon nano tubes (CNTs) are cylindrical molecules that consist of rolled-up sheets of single-layer carbon atoms. They can be single-walled with a diameter of less than 1 nm or multi-walled, consisting of several concentrically interlinked nano tubes, with diameters reaching more than 100 nm. Their length can reach several micrometers or even millimeters.
Like their building block graphene, CNTs are chemically bonded with sp2 bonds, an extremely strong form of molecular interaction. This feature combined with carbon nano tubes natural inclination to rope together via van der Waals forces, provide the opportunity to develop ultra-high strength, low-weight materials that possess highly conductive electrical and thermal properties. This makes them highly attractive for numerous applications.
The rolling-up direction of the graphene layers determines the electrical properties of the nano tubes. Chirality describes the angle of the nano tube's hexagonal carbon-atom lattice.
Armchair nano tubes – so called because of the armchair-like shape of their edges – have identical chiral indices and are highly desired for their perfect conductivity. They are unlike zigzag nano tubes, which may be semiconductors. Turning a graphene sheet a mere 30 degrees will change the nano tube it forms from armchair to zigzag or vice versa.
While MWCNTs are always conducting and achieve at least the same level of conductivity as metals, SWCNTs' conductivity depends on their chiral vector: they can behave like a metal and be electrically conducting; display the properties of a semi-conductor; or be non-conducting. For example, a slight change in the pitch of the helicity can transform the tube from a metal into a large-gap semiconductor.
It is an allotropic form of the carbon atom (Allotropy form- When any element is formed in more than one form and in each form they posses different property). An Er. & Scientist Richard Buckminster Fuller extracted that carbon is having cluster the very first form gathered is C16.
Structure:
Fullerene is a soccer ball like structure which is hollow from inside and is polymorphic in nature (arrange in hexagon and pentagon form) These are arranged in manner that posses 2 hexagon share a common wall while 2 different pentagon never share the common wall. Each carbon atom is sp2 hybridized. Electric spark is produced at graphite ectrode at inert atmosphere and low pressure which gives back black soot that consist of fullerene and impurities. These impurities are removed by the method of sublimation.
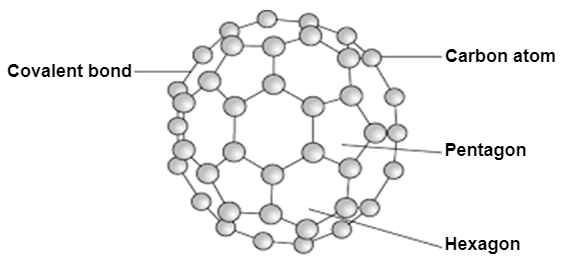
Properties:
All carbon atoms are sp2 hybridized with FCC in nature. This is mustard yellow in color. The fullerene may be Exohederal or Endohederal structure based on the occupying the space.
Applications:
1-Used as super conductors
2-Fulleren is used in making the Ferro magnets
3-It can be used for making non-linear electronic devices.