UNIT 4
INSTRUMENTAL METHODS
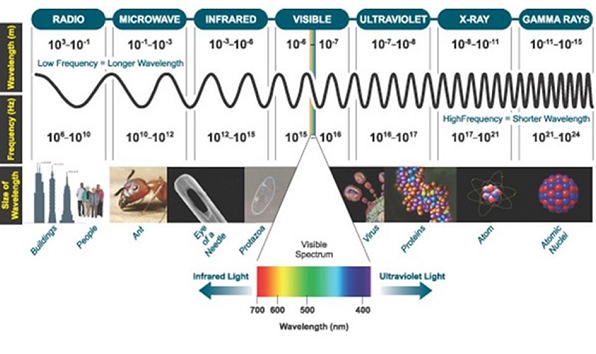
The distribution of electromagnetic radiation according to their frequency or wavelength. It is the range of frequencies, wavelength covering the frequencies below 1 hz to above 1025 hz. Generally, in vacuum electromagnetic waves tend to travel at speed of light. However, they do so at a wide range of wavelengths, frequencies, and photon energies.
The electromagnetic spectrum consists of a span of all electromagnetic radiation which further contains many sub ranges which are commonly referred to as portions. These can be further classified as infra-red radiation, visible light or ultraviolet radiation.
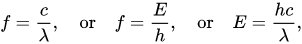
Type of Radiation | Frequency | Wavelength |
Gamma rays | 10 20- 20 24 | <10 -12m |
Ultraviolet | 10 15- 1017 | 400 nm- 1 nm |
Visible | 4 – 7.5*10 14 | 750nm -400nm |
x-rays | 10 17-10 20 | 1nm-1pm |
UV and visible radiation are energetic electromagnetic radiations that bring about electronic excitations.
The electromagnetic radiations with weakness range 10 – 400nm are called ultraviolet radiation and 400nm are called visible light radiations.
Lambert – beer laws of absorption
Lamberts law: -
The rate of decrease in intensity of radiation is directly proportional to path length of solution when the monochromatic light passes through a solution of constant concentration.
Mathematically the law can be written as,
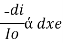
Therefore,
 intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.
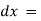 small thickness of solution or path length.
small thickness of solution or path length.
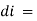 small decrease in intensity of light
small decrease in intensity of light 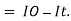
Intensity or radiant power is the number of photons per unit area per second
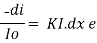
Integrating within limits
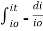 =
= 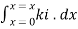
Therefore,(ln it – ln io) = ki. x
In io/it = ki.x
Lambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration.
Beer’s law: -
The rate of decrease in intensity of light proportional to concentration of solution for a fixed thickness of solution.
Mathematically the low can be written as,
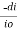 ά dc
ά dc
Integrating within limits
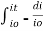 =
= 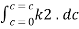
Therefore,
- -( ln it – ln io) = k2.c
- Or ln io/it = k2c
- It = io. e-k2.c
In other words, beers law can be stated as the intensity of transmitted light decrease exponentially with increase of concentration, for a fixed path length of solution.
Combined lambert – beers law
The combined lamberts – beers law will be
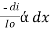 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration)
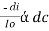 ----------------------- - - (beers law for constant thickness of solution)
----------------------- - - (beers law for constant thickness of solution)
Therefore,
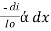 or
or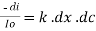
Integrating within limits
Ln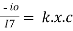
Or
 Log
Log 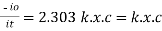
Therefore,
K is absorptivity constant
The term log 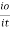 is defined as absorbance (a) and transmittance (T) is defined as it/io
is defined as absorbance (a) and transmittance (T) is defined as it/io
A= K.X.C Lambert – beers law
Therefore,
X is in om C is in gm/lit
Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.
By taking unit concentration of sample in solution (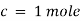 ) and unit length of path (
) and unit length of path (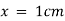 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.
A= E.X.C
Therefore,
E is known as molar absorptive or molar extinction coefficient.
Ultra-Violet Spectroscopy:
Ultraviolet and Visible Spectroscopy helps ion the determination of the absorbance spectra of the compound in the solution. The absorbance of light energy or the electromagnetic radiations are observed. This is responsible for the exciting the electron from the ground state to the first singlet excited state of a compound. The UV-Visible region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm.
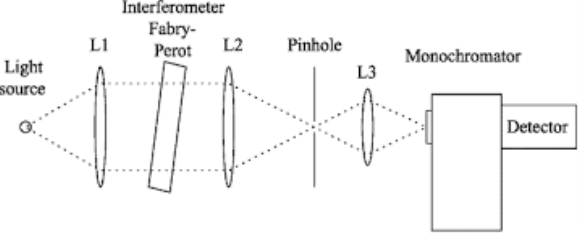
Monochromator used to measure the wavelength of the light. As the figure shows monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has a diode array detectors that allows the instrument to simultaneously detect the absorbacne at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
Applications:
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High performance liquid chromatography.
3- Ultraviolet-Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of wafer.
Infra-Red Spectroscopy:
The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Taking the molecule A-B in consideration then the one get partial negative while other will be partial positive charge. There are two different type of vibration that is Coupled vibration and De-Coupled Vibrations. The change in the relative position of 2 atoms is called as the coupled vibration while de-coupled vibration means 1 atom get fixed at a single position instead the other atom is in rotation. The vibration leads to the expansion and contraction of the atoms in the molecule. The change in the dipole movement due to the vibration then only those molecule exhibits vibrational spectroscopy.
Applications:
1-Infrared technique is used in organic and inorganic chemistry.
2-This is used in quality control, dynamic measurement.
3-This is majorly used in the forensic labs.
4-Infrared Spectroscopy is used in measuring the degree of polymerization in manufacturing the polymers.
Nuclear Magnetic Resonance:
NMR stands for the Nuclear Magnetic Resonance Spectroscopy. NMR Spectroscopy technique is used to observe local magnetic fields around atomic nuclei. An NMR instrument allows the molecular structure of a material to be analyzed by observing and measuring the interaction of nuclear spins when placed in a powerful magnetic field. For the analysis of molecular structure at the atomic level, electron microscopes and X-ray diffraction instruments can also be used, but the advantages of NMR are that sample measurements are non-destructive and there is less sample preparation required.
Nuclear magnetic resonance (NMR) spectroscopy is a crucial analytical tool for organic chemists. The research in the organic lab has been significantly improved with the aid of the NMR. Not only can it provide information on the structure of the molecule, it can also determine the content and purity of the sample. Proton (1H) NMR is one of the most widely used NMR methods by organic chemists. The protons present in the molecule will behave differently depending on the surrounding chemical environment, making it possible to elucidate their structure.
Chemical shift in NMR spectroscopy:
A spinning charge generates a magnetic field that results in a magnetic moment proportional to the spin. In the presence of an external magnetic field, two spin states exist; one spin up and one spin down, where one aligns with the magnetic field and the other opposes it.
Chemical shift is characterized as the difference between the resonant frequency of the spinning protons and the signal of the reference molecule. Nuclear magnetic resonance chemical change is one of the most important properties usable for molecular structure determination. There are also different nuclei that can be detected by NMR spectroscopy, 1H (proton), 13C (carbon 13), 15N (nitrogen 15), 19F (fluorine 19), among many more. 1H and 13C are the most widely used. The definition of 1H as it is very descriptive of the spectroscopy of the NMR. Both the nuts have a good charge and are constantly revolving like a cloud. Through mechanics, we learn that a charge in motion produces a magnetic field. In NMR, when we reach the radio frequency (Rf) radiation nucleus, it causes the nucleus and its magnetic field to turn (or it causes the nuclear magnet to pulse, thus the term NMR).
This is the one of the fastest separation method, which allows for the quantitative determination of each compound of the mixture. In the Gas-Liquid chromatography the process begins by the bringing of sample by the syringe to the heated injection chamber. The sample is passing through the nitrogen gas in the column. The same column is has the thin film of liquid. After the separation of the carried gas the separated mixture flows in detector which activates a recorder. Traces a series of peaks on chromatogram.
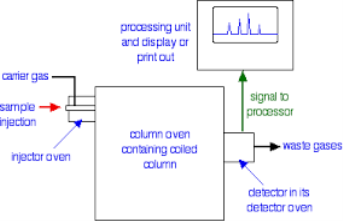
Principle:
Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase is separating from each other while moving with the aid of a mobile phase. The factors effective on this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights because of these differences, some components of the mixture stay longer in the stationary phase, and they move slowly in the chromatography system, while others pass rapidly into the mobile phase and leave the system faster.
Instrumentation and Applications
1) The chromatographic technique is used for the separation of amino acids, proteins and carbohydrates.
2) It is helpful for the qualitative and quantitative analysis of complex mixtures.
3) It is also used for the analysis of drugs, hormones, vitamins and brain amines.
4) This technique is also helpful for the determination of molecular weight of proteins.
5) It is helpful in detection of phenolic compounds in drinking water.
6) It is helpful in detection of endogenous Neuro peptides in extracellular fluid of brain etc.
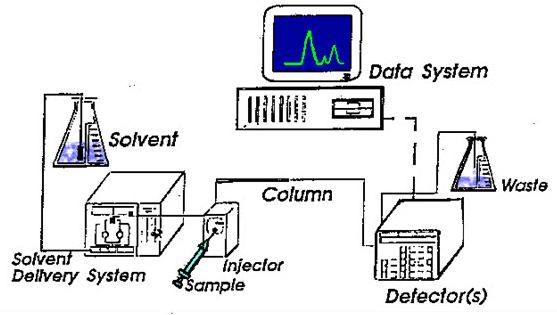
It stands for High Performance Liquid Chromatography. It is the method that is used to separate a mixture of compounds in analytical chemistry into the individual components of the mixture. This mixture can be separated by using the basic principle of column chromatography and hence after that that is identified and quantified by spectroscopy.
Working & principle:
The mixture could be taken in very small amount that to be separated and tested is sent to stream of mobile phase percolating by the help of column. The separation took place in a separation column between a stationary and a mobile phase. The stationary phase is a granular material that too with a very small porous particle in a separation column while there is the mobile phase which forces the solvent mixture at high pressure through the separation column. Via a valve with a connected sample loop, i.e. a small tube or a capillary made of stainless steel, the sample is injected into the mobile phase flow from the pump to the separation column using a syringe. Subsequently, the individual components of the sample migrate through the column at different rates because they are retained to a varying degree by interactions with the stationary phase. After leaving the column, the individual substances are detected by a suitable detector and passed on as a signal to the HPLC software on the computer. At the end of this operation, a chromatogram in the HPLC software on the computer is obtained. The chromatogram allows the identification and quantification of the different substances.