UNIT-1
High Polymers
Definition
A polymer is a large molecule with high molecular weight, this high molecular weight is obtained when smaller molecules having low molecular weight of one or more types undergo the chemical interaction. The process of manufacture of a polymer is known as polymerization.
Polymer, either they are natural or synthetic substances consists of very large molecules called macromolecules, these macromolecules are units or multiples of simpler chemical units called monomers. Polymers find their space in many of the materials in living organisms, including, for example, proteins, cellulose and also nucleic acids. They are also present in manmade materials such as concrete, glass, plastics and rubbers, polymers also constitute the basis of such minerals like diamond, quartz and feldspar.
The word polymer designates an unspecified number of monomer units. When the number of monomers is very large, the compound is sometimes called a high polymer
Types of Polymerisation and Mechanism.
The repeated units of monomers form the basis for the formation of, these are linked together by covalent bonds. The reaction leading to the formation of with their respective monomers is called polymerization.
Addition polymerization:
In Addition, polymerization, molecules of the same or different monomers add up together to form a polymer on a large scale. When monomers are being added to form a large chain such a process is known as chain growth polymerization. The monomers that are used in this type of polymerization are unsaturated compounds (the compounds of carbon are connected by double or triple covalent bonds).
- For example, alkenes, alkynes and alkadienes.
The chain length is increased either by the formation of ion species or free radicals, in this case however the reaction takes place in the presence of free radicals, the mechanism is discussed below
Free radical mechanism:
Free generating initiator are the catalysts that polymerize the reaction of the compound of alkenes and their derivatives.
- For example, benzoyl peroxides.
When ethene is polymerised to polythene, the mixture of ethene is first heated with a small amount of the initiator benzoyl peroxide. A new and large free radicle is formed, when there is an additional free radicle formed by peroxide to the double bond of ethane. This step is known as the chain initiating step, this means that the chain formation is initiated in this very step. As the process proceeds, a bigger sized radical is formed as the free radical obtained from the first step reacts with another molecule of ethane. A chain propagating step is formed as the reaction continuous with the sequence being repeated leading to the formation of bigger and new radicals. The last stage being the chain termination step, where the radical formed and the product radical react with another radical to form the final polymerized product, and hence this is called the chain terminating step.
2. Condensation polymerization:
This type of polymerization involves a repeated condensation process between bi-functional monomers. Simple molecules such as water and alcohol are lost due to the condensation process. The Mechanism of Condensation mechanism refers to combining of smaller molecules to get larger molecules. The product that is formed in this polymerization is a bi-functional species, these species will further undergo a sequence of processes, however at each step a very unique and distinct functional species and each species that is forms is independent when compared to the other species. Hence this process is called as step growth polymerization.
- Examples, include ethylene glycol and terephthalic acid.
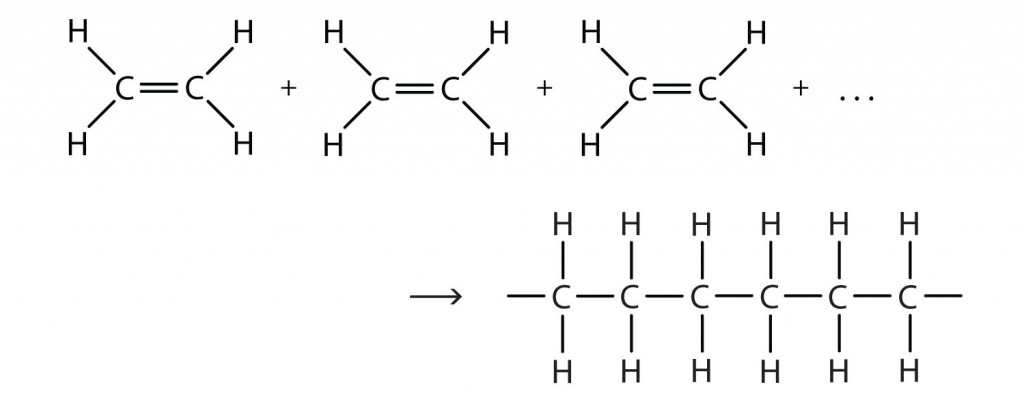
Polymerization Reaction
Karl Ziegler, a German chemist in the early 1950s, he was successful in discovering a method for making an entire linear HDPE, at a very low temperature and pressure in the presence of complex organometallic catalysts. By successive insertions of ethylene molecules, the polymer chain grows from the surface of the catalyst, as shown in figure. Eventually the polymer chain detach itself from the catalyst surface once polymerization is complete. Among the variety of catalysts used, the most commonly used ones are titanium trichloride formed by combining transition metal compound.,TiCl3, is combined with an organo-aluminium compound such as triethylaluminium, Al(CH2CH3)3.
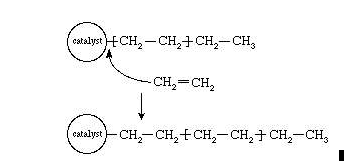
Fig2: The polymerization of ethylene (CH2=CH2) using a complex organometallic catalyst.
Immediately after the discovery of Ziegler, Giulio Natta who was an Italian chemist along with his co-workers had discovered that Ziegler-type of catalysts can polymerize propylene, CH2=CHCH3, that results in a polymer that holds the same spatial orientation and all the methyl (CH3) groups attached to the polymer chain:
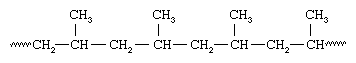
As all the groups of methyl are present on the same side of the chain, Natta called the isotactic polymer as polypropylene. Natta was also successful in synthesizing polypropylene having the methyl groups in the presence of vanadium-containing catalysts, these were oriented the same way on alternate carbons hence the arrangement was called syndiotactic.
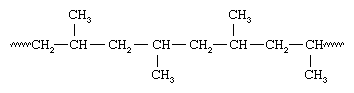
Stereo regular polymers also include Isotactic and syndiotactic polymers. These usually have an ordered arrangement of pendant groups along the chain. Atactic polymers are those where the orientation of groups is random. Stereoregular polymers have high degree of crystallinity and high tensile strength, they are usually uniform in nature due to the close packing of polymer chains.
Properties of Polymers
Physical Properties
- The tensile strength of the polymer increases with the increase in chain length and tensile strength.
- Polymers do not melt; they change their state from crystalline to semi-crystalline.
- Many small molecules called monomers form polymers through a process called polymerization, polymers can either be natural or synthetic. Their consequently large molecular mass relative to small molecules, produce unique physical properties that include toughness, high elasticity viscoelasticity, and a tendency to semicrystalline and amorphous forms rather than crystalline form.
Mechanical Properties
With some basic mechanical properties of the material before its application in any field, such as how much it can be stretched, how much it can be bent, how hard or soft it is, how it behaves on the application of repeated load and so on. a. Strength: In simple words, the strength is the stress required to break the sample. There are several types of the strength, namely tensile (stretching of the polymer), compressional (compressing the polymer), flexural (bending of the polymer), torsional (twisting of the polymer), impact (hammering) and so on. The polymers follow the following order of increasing strength: linear < branched < cross-linked < network. Factors Affecting the Strength of Polymers
1. Molecular Weight: with the increase in molecular weight the tensile strength of the polymer increases, and reaches the saturation level is reached at some value of the molecular weight. When the molecular weight of the polymer is low, the polymer chains are loosely bound by the weak van der Waals forces and therefore the chains can move easily, and are also responsible for low strength, although crystallinity is present. When the molecular weight is large, the chains get entangled and become large and thereby give strength to the polymer.
2. Cross-linking: The strength of the polymer increases as the cross-linking controls the motion of the chains.
3. Crystallinity: The crystallinity of the polymer increases strength, because in the crystalline phase, the intermolecular bonding is more significant. Hence, the polymer deformation can result in the higher strength leading to oriented chains.
Plastics are compounds that have a wide range of synthetic or semi-synthetic organic compounds that are easily malleable and can be moulded into any solid form. Plastics are typically polymers that have molecular mass and are organic in nature, and often contain other substances also. They are usually synthetic in nature, most commonly derived from petrochemicals. However, an array of variants is made from renewable materials such as polylactic acid from corn or cellulosic from cotton linters.

Fig 3: The plastic handle of a spatula that has been deformed by heat.
One important classification of plastics is by the characteristic of permanence or impermanence of their form, or whether they are: thermoplastics or thermosetting polymer.
Thermoplastics these are plastics that do not show any changes in their chemical composition when subjected to heating, therefore they can be moulded again and again. Examples include: PVC, polystyrene and polypropylene.
Thermosets, or thermosetting polymers, when heated will melt and will take shape only once it cannot be moulded any further once they are solidified. If they are heated again, they do not melt but decompose. An irreversible chemical reaction occurs in the thermosetting process. A good example of thermosetting polymers is vulcanization of rubber is an example of a thermosetting process.
Compounding
Compounding is a procedure that provides formulations for the formation of plastics. The formulations consist of mixing or blending polymers that are in a molted state to attain the needed characteristics. Through hoppers the blends doses are automatically fixed at set points the blends usually are ABS, SMA, SAN. Strengthening agents like glass fibres are added if characteristics. Through-stabilizers and anti-oxidants are the additives that are added.
Compounding is usually done by extrusion the process begins with a hopper where the resins are added to the dye gradually. There are different zones in the barrel that can be modified according to the properties of resin.
The most common compounders used in the plastic industry are the Co-kneaders and twin screws (co- and counter rotating) as well internal mixers.
The process proceeds to form extrudates, which are land plastic threads or strands, these are then cooled in a water bath or sprayed on a conveyor belt, which then moves into a granulator, in the granulator the strands are broken down to obtain the desired pellet sizes.
The manufacture design and assembly of plastic is done by one of the methods.
 Fig 4: Plastic bottles: Plastic fabrication products
Fig 4: Plastic bottles: Plastic fabrication products
Plastic Fabrication Methods
The property of plastic, that include cost efficiency and its malleability, can make it a durable product for a wide range of products.
Plastic Welding
Plastic welding includes heating the substance to melt two or more pieces together. The thermoplastics which are known to be unsuitable for adhesive binding is effective for this process.in this process the individual pieces are fused or joined together with a filler material between them, this is done when the pieces differ extremely in the boiling point. Welding is accomplished by different methods like high frequency vibration, spinning or hot gas.
Compounding (or Blending) Plastic Fabrication Techniques
This process involves combining two or more plastics to form an amalgam, which later is formed into a single part. In this process, the plastic that is in molten form is mixed with exact specifications which eventually forms them into a mould, dye or any other tool.
Compounding is often used to improve the ease of processing a given material or to enhance product performance. By combining the advantages and disadvantages of several types of plastic, the process can result in a unique material complementary to a specific application. Some common types of plastic compounds include:
- Polymer fillers
- Base resins
- Pigment master batches
- Blowing agents
- Flame-retardants
- Purge compounds
Plastic Lamination
Lamination in plastics creates a barrier on another material along its surface. Lamination is done to improve certain features in the product such as styling, durability or the products aesthetic quality. Lamination also helps in reducing the cost of the material, in cases the material is sensitive or is prone to destruction, lamination helps in shielding the defects and reduces its need for maintenance.
Two most common types of lamination are the film and resin. In both processes, for a moving substrate, heat and pressure are applied for adhesion to a fabricated film the most effective method for forming a plastic barrier on the exterior of the product is the film lamination method., while resin lamination is more frequently used to create an adhesive layer between two substrates. Paper, fabrics, metal sheeting, and flexible foam are common lamination base materials.
Molding Processes
In plastic moulding, plastic is formed into a specified shape by allowing the heated, pliable work piece to cool and harden around or within a mold. There are various methods for plastic molding methods. They are blow moulding, injection molding and rotational molding. Rotational molding is utilised in hollow products that are plastic in nature, they include toys, buoys and automotive parts, products such as fuel tanks or bottles are done by Blow molding is often used to create containers, , while injection molding is used in large scale production like dishware that need a higher melt index, like dishware production.
Plastic Extrusion
Plastic Extrusion is a process that can be used to prepare tubing, piping, or components used for sheeting. The Extrusion process is utilised to further enhance the processing stages. For example, plastic extrusion is often a precursor to adhesion or lamination procedures.
Profile extrusion and sheet extrusion are the most common forms of the process. profile extrusion is a process where the plastics pellets are melted using a single screw extruder, the melted plastic is moves through a pressurized screw mechanism and forms an annual dye by pressure. Very thin plastic sheets are obtained through this method, the plastic formed solidifies around a calibration sleeve to create a pipe or tube component of a specific diameter. The Sheet extrusion method, also uses a similar technique to create thin plastic sheeting.
Plastic Foaming
A variety of different shapes are formed from Foam products. Configurations of Common foaming configurations include sheet, round, film, solid plank, rod and bun stock. To achieve the desired characteristics, polymer composites are typically shaped through a process of physical or chemical blowing.
Choosing a Plastic Fabrication Process
Product functionality and ease of manufacturing are important things to consider when choosing a plastic fabrication process. Some methods are inefficient for fabricating certain types of plastic, and therefore may not be helpful for your particular project. Some other issues to keep in mind include:
- The need for single plastics versus plastic compounds
- The intended proportion of plastic to non-plastic material in the product
- The role of plastic in your fabrication process (as adhesion, lamination or base product)
- The dimensions and use of the final product,
(abbreviated PE; IUPAC name polyethene or poly(methylene)) is one of the most commonly used plastics today. It is a manmade plastic, that is an additional linear polymer. It is used in packaging as it is a homopolymer. The common examples are plastic bags, plastic films, geomembranes etc.
The properties of polyethylene can be divided into mechanical, chemical, electrical, optical, and thermal properties.[13]
Mechanical properties of polyethylene
Polyethylene mechanical properties include hardness and rigidity and is of low strength, they also have a high ductility, low friction. It shows waxiness, they are strong creep when subjected to pressure and force that can be reduced by short fibres.
Thermal properties
The commercial applicability of polyethylene is limited by its low melting point compared to other thermoplastics. The melting point typically ranges from 120 to 130 °C (248 to 266 °F) for polyethene’s that have common commercial grades of medium- and high-density polyethylene. On the other hand, the melting point is typically ranging from 105 to 115 °C (248 to 266 °F) for polyethene’s that are commercial, and have low-density. The temperatures vary depending upon the type of polyethylene., but the theoretical upper limit of melting of polyethylene is reported to be 144 to 146 °C (291 to 295 °F).
Chemical properties
Polyethylene has its chemical properties similar to paraffin, polyethylene has high molecular weight, nonpolar and saturated, hydrocarbons. They have a symmetric structure therefore the single macromolecules are not linked covalently. Thus, they are partially crystalline in nature, the higher the crystallinity more will be the stability.
Most polyethylene’s varying with respect to their grades have, are not easily attached by strong acids or strong bases, they are also resistant to gentle oxidants and reducing agents, however at room temperature polyethylene do not dissolve.
Polyethylene do not absorb any water, oxygen, carbon dioxide can pass it easily. The permeability is low in case of plastics for gas and water vapour.
PE when exposed to sunlight become brittle.
Polyethylene cannot be imprinted or bonded with adhesives without pre-treatment. High strength joints are readily achieved.
Electrical properties of polyethylene
Polyethylene offers good electrical resistance and is also good electrical it is easily subjected to charging electrostatically, but is reduced by addition of carbon black and graphite.
Optical properties
The optical properties vary from clear, to milky opaque to opaque. LDPE has the greatest, LLDPE slightly less and HDPE the least transparency. Transparency is reduced as they are larger than the wavelength of visible light.
Polymerization
Polymerization of ethylene to polyethylene is described by the following:
n CH
2=CH
2 (gas) → [–CH
2–CH
2–]
25.71 ± 0.59 kcal/mol (−107.6 ± 2.5 kJ/mol)
Ethylene is a stable compound and highly exothermic in nature they polymerize only when they come in contact with catalysts. The most common catalysts consist of Titanium III chloride, the so-called Zeigel-Natta catalyst etc. Polyethylene can be produced through radical polymerization.
The physical properties include
They are brittle and solid in nature and insoluble in Alcohol, but soluble in Hydrofluran,
They show high amount of Hardness and show thermoplasticity, as the mechanical properties increase with the Increase in temperature.
Heat Stability of PVC is quite poor as they begin to decompose once the temperature reaches 140 °C, the melting point of PVC is 160 °C.
The elasticity of PVC reaches to 1500-3000 Mpa. They have ordinary friction.
PVC shows good insulation properties, but its insulating property is less than that of Polyethylene.
As the dielectric constant, dielectric loss tangent value and volume resistivity are high, the corona resistance is not very good, it is generally suitable for medium or low voltage and low frequency insulation materials
Chemical structure:
H Cl
| |
– C – C –
| |
H H
PREPARATION:
Poly vinyl chloride is prepared by polymerisation of monomer vinyl chloride
– About 80 percent of polymerisation includes suspension polymerisation.
-12% emulsion polymerisation and 8% bulk polymerisation.
Process: In goes the VCM and water are into the reactor and a polymerization initiator, along with other additives. The pressure in the vessel is kept tight to keep the VCM. To ensure a uniform particle size of the PVC resin, they are continuously mixed. The reaction is exothermic, and thus requires cooling. The suspension needs to be in its form therefore water is added as PVC is denser than VCM.
The polymerization of VCM is started by the droplets being mixed by compounds called initiators, the radical chain reaction begins when these initiators break down. Some examples of initiators include dioctanoyl peroxide and diacetyl peroxydicarbonate, both of which have fragile O-O bonds .to bring about a uniform rate to a polymerization reaction, a combination of initiators is used, as some initiators start the reaction rapidly but later slow down while others show the opposite therefore a combination of initiators are used to bring a steady rate to polymerization reaction. One the polymer grows to a size of 10x, the short polymers that are formed precipitate inside the droplet of VCM, and the process continues with the precipitated particles that are solvent swollen in nature.
On the course of the reaction, a PVC slurry is formed which is subjected to degas and stripping to remove excess VCM which is also recycled. Later the polymer is then passed through a centrifuge to remove water. The slurry is further dried in a hot air bed, and the resulting powder sieved before storage or pelletization. Normally, the resulting PVC has a VCM content of less than 1 part per million.
Bakelite is the commercial name for the polymer obtained by the polymerization of phenol and formaldehyde. These are one of the oldest polymers that were synthesized by man. Phenol is made to react with formaldehyde. The condensation reaction of the two reactants in a controlled acidic or basic medium result in the formation of ortho and para hydroxymethyl phenols and their derivatives.
Preparation and Properties of Bakelite
In a reaction when Phenol is present in excess, the reaction becomes acidic making the end product also acidic, however, when formaldehyde is added in more quantity than phenol the reaction becomes basic and the end product becomes basic in nature and is known as Resol.
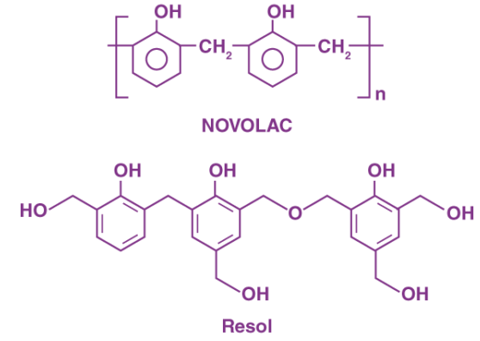
Fig 5: Structures of Resol and Novolac
There are intermediate products formed during the process of condensation and these are used as resins in the industries These intermediate condensation products are used as resins in different industries. When the compound Novolac undergoes cross linking with the help of phenol, which acts as a cross linking agent. Bakelite is produced.
The preparation of Bakelite involves several steps, as illustrated below.
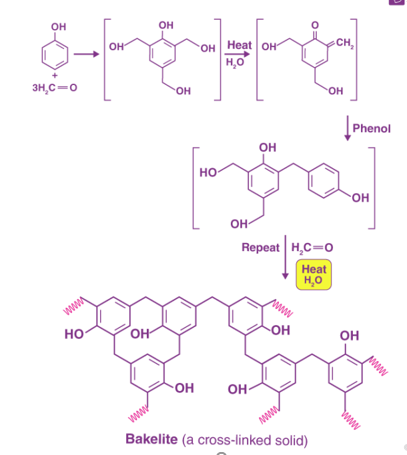
Fig 6: Preparation of Bakelite.
Initial methods of preparing Bakelite involved the heating of formaldehyde and phenol in the presence of one of the following catalysts -zinc chloride (ZnCl2), hydrochloric acid (HCl), or ammonia (NH3).
Bakelite Structure
The cross-linked product of phenol and formaldehyde has the following structure.
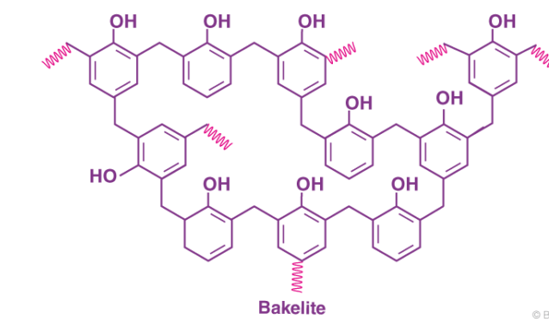
The chemical formula of Bakelite can be written as (C6H6O-CH2OH) n.
Properties of Bakelite
Some important properties of Bakelite are listed below.
- Bakelite can be quickly molded.
- The smooth moldings can be obtained with this polymer.
- Bakelite moldings are heat-resistant and scratch-resistant.
- Bakelite is also resistant to several destructive solvents.
- Owing to its low electrical conductivity, Bakelite is resistant to electric current.
Elastomers are polymers that are having a viscosity as well as elasticity and therefore are known as viscoelasticity. The molecules of elastomers inherit the property of regaining its original shape and size even after being stretched to a grate extent. The molecules are held together by weak intermolecular forces; however, they show high strength yield or strain in failure.
Rubber, is a substance which is elastic in nature and is obtained from the sap of certain tropical plants, they are also derived from petroleum of natural gas (synthetic rubber). The unique properties of rubber are that they are Elastic, tough and shows resilience, rubber forms the basic constituent of tyres used in automobile vehicles, bicycles and aircraft. Automobile is the larger consumer of Rubber in the form of tyres, the rest of the rubber is utilised in making mechanical parts such as gaskets, belts, hoses, furniture, clothing and toys. The main chemical constituent of rubber are elastomers, or “elastic polymers,” large chainlike molecules that can be stretched to great lengths and yet recover their original shape. The first Elastomer discovered is polyisoprene, from this natural rubber is manufactured, Hevea brasiliensis is the predominant tree located in Brazil. The first name Rubber was given by Joseph Priestly, as he found it useful in robber pencil marks. The major breakthrough came when it was discoveries after vulcanization process discovered by Charles Goodyear in 1839. Natural rubber is found in the sap present on the bark of many tropical and sub-tropical shrubs and trees.
Among the most important synthetic Rubber are styrene-butadiene rubber, neoprene, butadiene rubber, polysulfide rubber and silicones. Synthetic rubbers, like natural rubbers, can be toughened by vulcanization and improved and modified for special purposes by reinforcement with other materials.
Vulcanization, is a process natural rubber is treated with sulphur, the physical properties of both natural and synthetic polymers are improved. The rubber that has undergone vulcanization shows higher tensile strength and becomes resistant to abrasion or swelling,
Vulcanization is defined as a process of curing elastomers, in the process cross linking are formed between sections of polymer chains, thereby increasing their durability and rigidity. Changes are also brought about in the electrical and mechanical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible.
The word vulcanization is derived from Vulcan, the Roman god of fire and forge.
This is a copolymer that is produced from a combination of Styrene and butadiene, they are nothing but synthetic rubbers. And are known to be utilized more than any other synthetic rubber. They replace the natural rubber (polyisoprene) and are used in large scale in automobile and truck tires.
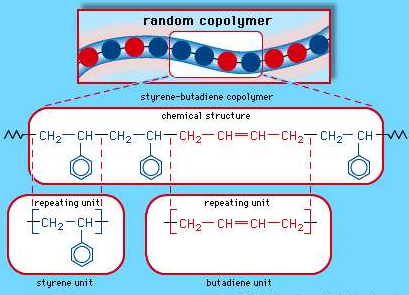
The random copolymer arrangement of styrene-butadiene copolymer.
Each coloured ball in the molecular structure diagram represents a styrene or butadiene repeating unit as shown in the chemical structure formula.
SBR is known to have good abrasion resistance, they are resistant to cracks and do not undergo ageing soon., They degrade by atmospheric Oxygen and ozone and is weakened by hydrocarbons. SBR replace the natural rubber due to their economic utilities.
T – THIOKOL RUBBER
POLYSULFIDE
Polysulfides, also called polythioethers or Thiokols (T), are compounds with thioether functions in the backbone. They are typically liquid polymers that can be crosslinked by oxidizing the polymer’s terminals thiol groups (-SH) to disulfide (-S-S-) links. Common curing agents are oxygen donating compounds such as manganese dioxide, cumene hydroperoxide, and p-quinone dioxime. Other organic hydro peroxides, aldehydes and metallic paint driers can also function as curatives.
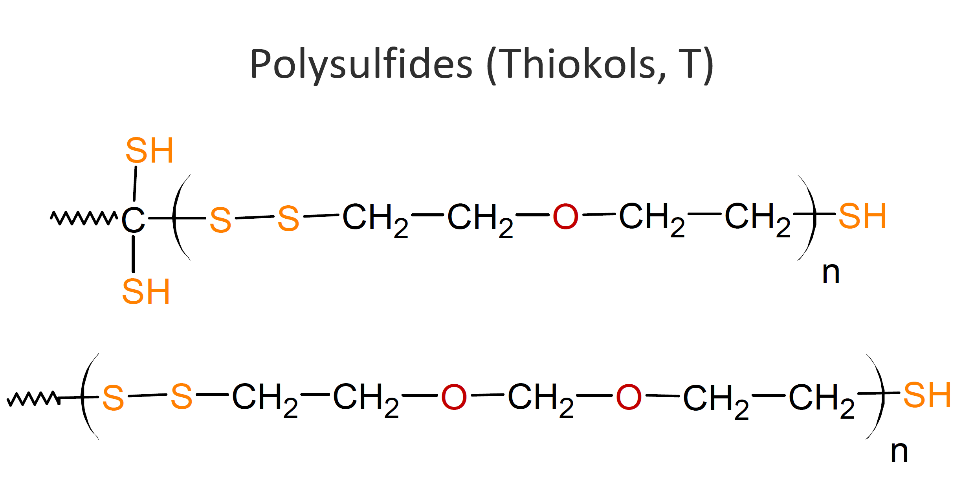
Thiokol elastomers show good resistance to oil, oxygen and ozone and other chemicals like aromatic hydrocarbons they exhibit gas permeability, and resistant to cracks. However, polythioethers have some major limitations. They do not stand out with respect to performance when compared to other elastomers, they show very poor resistance to heat, low tear. They are also not resistant to many polar solvents including (fatty) esters, mercaptans, amines, and chlorinated hydrocarbons.
APPLICATIONS
Polysulfides are widely used as the base polymer for sealants, caulks, and adhesives in various applications within the building & construction, aircraft, and automobile industry. Other industrial applications include highways sealants, molding / potting compounds, and concrete coatings.
The typical working temperature range is -45°C to +105°C. However, some grades can withstand temperatures up to 150/170°C (for a short time).
Fibre-reinforced plastic (FRP) (also called fiber-reinforced polymer, or fiber-reinforced plastic) is a polymer matrix made of composite material that is reinforced with fibres. The fibres are usually basalt or aramid, they are usually fibre in fibreglass and carbon in carbon reinforced polymers. In rare cases other fibres like paper, wood or asbestos may be used. Though resins that consist of phenol formaldehyde are still used. The polymer is usually a vinyl plastic, epoxy.
FRPs are commonly used in the aerospace, automotive, marine, and construction industries. They are commonly found in ballistic armor as well.
These polymers can be decomposed by microorganisms or enzymes under conditions like aerobic or anaerobic, the polymers under such conditions develop into starch cellulose and polyesters. Aliphatic polyesters are the most commonly used polymers of this type.
Biodegradable Polymers
Some examples are given as follows: -
Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV): It is derived by combining 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid, in which monomers are cross-linked by an ester linkage. It decomposes to form carbon dioxide and water. The compound is brittle and is utilised in drug production and bottle manufacture.
A polymer that can be decomposed by bacteria is called a biodegradable polymer.
The biodegradable polymer are the polymers which are degraded by the micro-organism within a suitable period so that biodegradable polymers & their degraded products do not cause any serious effects on the environment. They degrade by enzymatic hydrolysis & oxidation. The decomposition reactions involve hydrolysis (either enzymatically induced or by non –enzymatic mechanism) to non- toxic small molecules which can be metabolized by or excreted from the body.
The common examples of aliphatic biodegradable polymers are polyglycolic acid(PGA), Polyhydroxy butyrate (PHB), Polyhydroxy butyrate’s-co-beta hydroxyl valerate( PHBV), Polycaprolactone(pcl), Nylon-2-nylon-6.
These polymers are used mainly for medical goods such as surgical sutures, tissues in growth materials, for controlled drug release, plasma substitutes etc. They are also used in agriculture materials, such as films, seed coatings, fast food wrappers, personal hygiene products etc.
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are polymers that are organic in nature and also conduct electricity., such polymers can be semiconductors or may show metallic conductivity. The conductive polymers show one of the biggest properties of dispersion, they are not thermoplastic in nature and have the advantage of being processable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. Methods like organic synthesis and dispersion techniques can be implemented to enhance the electrical properties in conductive polymers.
References :
- Principles of Polymer Chemistry by Paul.J.Flory
- Principles of Polymerization by Geroge odian.