UNIT -2
Corrosion
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the corrosion.
All metals can corrode. Some metals, like pure iron, deteriorate very fast. Stainless steel, and, metals that combines with iron and other alloys, is slower to corrode and is therefore used more efficiently.
All small group of metals, are called the Noble Metals, and show much less reaction than others. As a result, they deteriorate rarely. They are, in fact, the only metals that are found in nature in their purest form. The Noble Metals, not unexpectedly, are often very valuable. They include gold, rhodium, silver, palladium, and platinum.
Dry or Chemical theory of corrosion: The direct reaction of Atmospheric gases (like halogens, oxygen, oxides of nitrogen and sulphur, fumes of chemicals and with metals and hydrogen sulphide), on the surface of the metal causes corrosion. Oxygen is the most responsible for corrosion of metals than any other gases or chemicals. This process occurs in the absence of moisture and affects the metal surface directly. Corrosion products are formed at the site of corrosion.
The three types of corrosion are
Oxidation Corrosion, Liquid metal corrosion and Corrosion by other gases.
Wet or Electrochemical theory of Corrosion: This corrosion occurs in metals that come in direct contact with a conducting liquid of two different metals are dissolved in a solution partly. There is formation of galvanic cell on the metal, as part of the metal acts as an anode and the rest is the cathode, the chemical in the solution along with the humidity acts as the electrolyte. Oxidation takes place in such conditions resulting in corrosion at the anode surface and reduction occurs at the cathode surface of the metal. In this case corrosion occurs at the anode but rust gets deposited on the cathode.
1. The more the metal shows its reactivity, the possibility of the metal getting corrode increases.
2.The corrosion occurs faster when the impurities help in setting up voltaic cell.
3. The rate of corrosion is also affected by the presence of electrolytes in water.
4. The rusting of Iron is increased if the amount of carbon dioxide is present in more amounts in water.
5.The rate of corrosion can however be reduced when the surface of Iron is coated with layers of metal that are actually more active than the Iron itself.
6. An increase in temperature (within a reasonable limit) also increases the rate of corrosion.
Corrosion is a serious problem faced whenever there is metal material which is placed at any location. When a metal is exposed to humidity or other water sources, or if the metal is present under water, corrosion occurs.
Cathodic Protection
Cathodic protection in this process the main principle is to switch from the works by changing over undesirable anodic (active) sites on to a metal's surface to cathodic (passive) destinations in the presence of a restricting current. This restricting current provides free electrons and also supplies power to the nearby anodes,
Cathodic protection can, the presence of galvanic anodes. This process is also known as conciliatory form, this technique utilises metal anodes together with the electrolytic condition, to make themselves strong (erode) so as to secure the cathode. This technique proves to save the metals galvanic corrosion.
Though the metal that requires protection can differ, the conciliatory anodes are largely made up of magnesium, or zinc, metals that have the most negative electro-potential. The arrangement of galvanic setup gives a glimpse of the distinctive electro-potential - or integrity - of metals and amalgams.
In a conciliatory framework, the anode should be supplanted regularly as the metallic particles move from the anode to the cathode, which drives the anode to erode more rapidly. The second technique for cathodic security is alluded to as awed current protection.
This strategy, which is regularly used to ensure covered pipelines and ship bodies, requires an elective wellspring of direct electrical current to be provided to the electrolyte.
Sacrificial Anode
It is a method where an easily eroded material is deliberately placed in a tank or a pipe to be sacrificed for corrosion and the rest of the system remains free from corrosion.
A sacrificial anode is also known as a galvanic anode
The mechanism that occurs in this process is very similar to that of an electrochemical system. In this technique the metal that is protected is placed on the cathode side and then a more reactive metal or alloy (having a larger potential difference than the protected metal) is chosen and connected to the protected metal as an anode. The redox reaction will proceed spontaneously. As the reaction proceeds the sacrificial metal gets consumed as oxidation reaction occurs at the anode, simultaneously reduction reaction occurs on the cathode, thus preventing the metal from erosion. Thus, corrosion on the protected metal is successfully shifted to the anode, protecting the metal.
Sacrificial anodes are normally supplied with either lead wires or cast-m straps to facilitate their connection to the structure being protected. The lead wires may be attached to the structure by welding or mechanical conn
The materials used for sacrificial anodes are either relatively pure active metals, such as zinc or magnesium, or are magnesium or aluminium alloys that have been specifically developed for use as sacrificial anodes.
Advantages of using sacrificial anodes:
- Can be used where there is no power
- Lower initial cost
- Less supervision required
- Comparatively simple installation and additional anodes can easily be added if the initial installation proves to be inadequate
Sacrificial anodes are used to protect:
- Hulls of ships
- Water heaters
- Pipelines
- Distribution systems
- Above-ground tanks
- Underground tanks
- Refineries
The anodes in sacrificial anode cathodic protection systems must be periodically inspected and replaced when consumed.
Impressed current methods
In this technique, protection from corrosion is obtained by electrochemical means. It is a type of cathodic protection, where the dissolution of anodic structures is reduced, that occurs by decreasing the difference the potential energy, and also when the metal surface is placed in a conductive electrolyte such as water and the cathodic and anodic metal surface sites face less reduction.
Theoretically, on observation the impressed current cathodic protection is obtained during the stage where open circuit potential of cathodic areas gets polarized into the same circuit potential of anodic sites. The key in impressed current protection is to turn the whole structure cathodic in nature, or make it a current receiver rather than a current provider.
An impressed current protection system can offer protection to structures and metals like:
- Fuel pipes
- Steel
- Water
- Hulls
- Tanks
- Water heaters
- Oil casings
It can also serve as a metal reinforcement in the case of concrete buildings, structures and bars. The method can be applied in galvanised steel, where the steel parts are protected from rusting by sacrificial zinc coatings.
Utilizing this type of protection offers the following practical benefits:
- Infrastructure cost for corrosion is reduced.
- Infrastructure failure incidents become rare.
- Product loss from corrosion is reduced.
- Lower downtime in terms of emergency repairs
- Increased service life of infrastructures
- Enhanced quality of infrastructure service
- Increased security and health to underlying infrastructures
An impressed current system serves as an ideal protection system for bigger structures that are not capable of delivering ample current to offer protection. This system is mainly composed of anodes attached to a power source (DC), which can be a rectifier connected to power (AC). In the absence of an AC power supply, other power sources may be used like wind power, solar panels and gas power. The anodes in the impressed current protection system can be found in various sizes and shapes. The most common ones are tubes and solid rods, while there are also continuous ribbons made from various materials like:
- Platinum
- Graphite
- Silicon
- Cast iron
Surface Coating
This is the most economical method and simple wherein the membrane surface is coated by a layer. This method can be carried out on a large scale in industries.
The most common coating materials include tin, lead, aluminium etc. The principle in this method includes formation of a transition layer from the various alloy compositions. To the substrate inter-metallic compounds of the two metals are normally present and on the exterior are alloys that consist of solid solution alloys that predominantly form the coating materials.
Surface coating is a process where a substance is applied to other materials bringing about changes like gloss, colour, resistance to chemical attacks or permeability, however the bulk properties of the material remains unchanged. Production of surface coating by any method depends primarily on two factors: the cohesion between the film forming substances and the adhesion between the film and the substrate. The development of science and technology revolutionized the surface coating industry
(Hot dipping, galvanizing, tinning, cladding, electroplating, electro less platting
- Hot-dip galvanizing is a form of galvanization where the iron or steel is immersed in a bath that consists of molten zinc at a temperature around 449o C (840 °F), this helps in producing a multi-layer coating of zinc-iron alloy and zinc metal. The layer is also corrosion resistant, when steel is immersed in zinc, a reaction which is metallurgical occurs between the iron and the molten zinc.
- Galvanizing, it is a process that involves the protection of steel and iron from exposure to the atmosphere leading to rusting, this is however prevented by the application of zinc coating. Proper coating by galvanization can protect the material from the atmosphere and corrosion for nearly 15 to 30 years, however is pores develop in the coating galvanic or electrolytic action occurs.
- Tinning involves coating sheets of steel with tin or wrought iron, the resulting plate is called as tinplate. Tinning also involves coating a metal with solder before it is subjected to soldering
This method is also used to apply to the ends of the stranded wires that are used as electrical conductors to stop oxidation, they are also used predominantly to control rust. When tinning is applied to stranded wires it prevents them from fraying or unravelling during twisting, binding posts, or wire connections that any cause short circuits.
- Cladding is done mostly in constructions, where a thin skin or layer is applied over the other layer, to provide thermal insulation and also resistance to weather. This method also improves the appearance of building. Cladding can be made of any of a wide range of materials including wood, metal, brick, and composite materials that can include aluminium, wood, blends of cement and recycled polystyrene, wheat/rice straw fibres. The cladding need not be waterproof but acts as a controlling element. Cladding can also be used to control noise and also act as a rainscreen to protect against the weather.
- Electroplating is basically the process of plating a metal onto the other by hydrolysis, that helps to prevent corrosion of metal. The method used is electric current that reduces dissolved metal cations, that forms a thin coherent metal coating on the *electrode. The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. Electroplating is also utilised in electrical oxidation of the anions, on a solid substrate like the formation of silver chloride on silver wire to form silver chloride electrodes.
It is also applied to modify the surface features of an object (e.g. Corrosion protection, lubricity, abrasion), but electroplating can also be used to build thickness or make objects by electro forming.
- Electroless plating, this process was discovered by A. Brenner and G. Riddle in 1944. This involves the plating of metals and does not use electricity. This is achieved by a process of controlled autocatalytic reduction that gives a uniform coating to plastics and metals. The principle of this method is the deposition of metals such as copper, silver, nickel and gold that is present on a variety of material by means of a chemical bath. This process is also used in mirroring, wherein a clear piece of glass is dipped in an ammoniacal silver solution mixed with Rochelle salt or with a nitric acid–cane-sugar alcohol solution. Non-metallic surfaces, such as plastics, must be chemically treated prior to electroless plating. The major expansion of electroless plating has come in the area of plastics, as in the plating of printed electronic circuits. A large number of consumer goods are coated by this method to create durable and attractive surfaces.
The branch which deals with the movement of energy from one form to the other and the relationship that exists between heat and temperature with energy and the work done is called as thermodynamics. Thermodynamics is a branch of Science that deals with the different forms of energy and work done in a system. Thermodynamics is only confined to Large scale response of a system which we are observed and measured in experiments
Energy:
It referred to the energy content that is present within the system. The energy represents the overall energy contained in the system and may include many forms of energy such as potential energy, kinetic energy etc. In a chemical reaction, we know the energy transformations and basic thermodynamics gives us information regarding energy change associated with the particles present in a system.
Factors affecting the internal energy
The internal energy of a system may change when:
Heat passes into or out of the system,
Work is done on or by the system or matter enters or leaves the system
Work:
Work done by a system is defined as the amount of energy exchanged between a system and its surroundings. Work is completely determined by external factors such as an external force, pressure or volume or change in temperature etc.
Heat:
Heat in thermodynamics is defined as the kinetic energy of the molecules of the substance or material. Heat and thermodynamics together form the basics which help process designers and engineers to optimize their processes and harness the energy associated with chemical reactions economically.
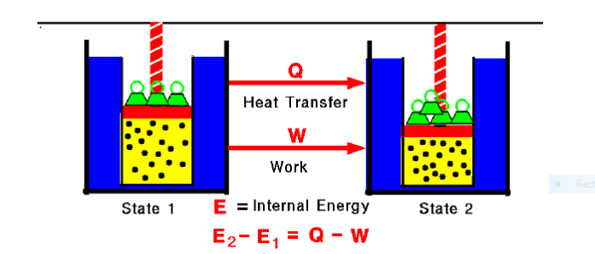
Fig. 1:
Any thermodynamic system in an equilibrium state possesses a state variable called the internal energy (E). Between any two equilibrium states, the change in internal energy is equal to the difference of the heat transfer into the system and work done by the system.
Enthalpy:
Enthalpy a process or activity takes place at constant pressure, the heat released or absorbed is equal to the Enthalpy change. Enthalpy is occasionally called or known as “heat content”, “enthalpy” is derived from the Greek word which means “warming”. Enthalpy(H) is the sum of the internal energy(U) and the result of pressure(P) and volume(V).
Enthalpy H is written as,
H = U + PVm
Where, H = is the Enthalpy of the system
U = is the Internal energy of the system and is entirely dependent on the state functions T, p and U.
Enthalpy can also be written as
ΔH=ΔU+ΔPV.
P = is the Pressure of the system
V = Volume of the system Enthalpy is not directly measured, but the change in enthalpy (ΔH) is measured, which is the heat lost or added by the system,
Entropy:
It was Introduced by the German Physicist Rudolf Clausius in 1850, and is a highlight of 19th century Physics. The word “entropy” is derived from the Greek word, which means “turning”. It was derived to provide a quantitative measure for the spontaneous changes and Clausius introduced the concept of entropy as a precise way of expressing the Second Law of Thermodynamics, Clausius form of the second law states that spontaneous change for an irreversible process in an isolated system
Is a measure of randomness or irregularity or disorder of the system?
The more or the randomness, higher is the entropy.
Solid state has the lowest or least entropy, the gaseous state has the highest entropy and the liquid state has the entropy that lies between the two.
Entropy is a state function. The changes in its value during any process, is called the entropy change.
ΔS = S2 -S1 = ∑S products – ∑S reactants
1) When a system absorbs heat, the molecules begin to move faster because the kinetic energy increases. Therefore, disorder increases. Greater the heat absorbed, greater will be the disorder.
2) For equal amount of heat absorbed at low temperature, the disorder will be more than at high temperature. This proves that entropy change is inversely proportional to temperature.
ΔS = eve / T
Entropy change during a process is defined as the quantity of heat (q) absorbed isothermally and reversibly divided by the absolute Temperature (T) at which the heat is absorbed
Free Energy
Gibbs Free Energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. It is a thermodynamic function that was discovered in 1876 by Josiah Willard Gibbs to assume if a process will occur spontaneously at constant temperature and pressure. Gibbs free energy G is defined as
G = H - TS
Where H, T, and S are the Enthalpy, temperature, and entropy. The SI unit for Gibbs energy is the kilojoule.
Gibbs free energy combines both the enthalpy and entropy into a single value.
Gibbs free energy is the energy t with a that associates itself with a chemical reaction. It equals the enthalpy minus the temperature of the product and the entropy of the system.
G=H-TS
At constant temperature
ΔG = ΔH – TΔS
ΔG predicts the direction of a chemical reaction. If ΔG value is negative, then the corresponding reaction is spontaneous. If ΔG value is positive then reaction is non-spontaneous.
ΔGº=ΔHº-TΔSº
Where
ΔGº = Gibbs free energy (J or KJ)
ΔHº=enthalpy
T=Temperature
ΔSº=Entropy
Gibbs free energy is the energy that is available to do quality work.
A reaction will spontaneously occur if ΔG<0 (exergonic reaction)
A reaction will not spontaneously occur if ΔG>0 (endergonic reaction).
If ΔG value is less than zero, there is a thermodynamic force for the reaction or it drives the process in the forward direction.
When ΔG is positive, then reactants are favoured, when ΔG=0 system is at equilibrium.
EMF
Electromotive force, or, as it is often written, e.m.f., is described as that source of energy which enables electrons movement around an electric circuit.
For any object to move from rest, there has to be some energy change. To ensure electrons movement round an electrical circuit, they should receive energy from a source of e.m.f. Which usually is a battery or a generator.
For every coulomb of electricity to move completely around an electrical circuit, a certain amount of electrical energy is needed, which depends on the particular circuit. The e.m.f. Is expressed in volts and is numerically the number of joules of energy given by the source of e.m.f. To each coulomb to enable movement around the circuit. The symbol for volt is the capital letter V.
Thus
Joules colombs=volts.
It follows that:
Joules=volts × coulombs = volts × ampers × seconds.
A 12-volt (12-V) battery is able to give 12 joules (12 J) of energy to each coulomb to enable movement around an electrical circuit.
The symbol for e.m.f. Is the capital letter E.
Example:
Calculate the energy supplied by a 12v battery when a current of 4 A flows for 10 minutes.
Energy supplied = Volts x amperes x seconds
= 12 x 4 x (10 x 60) Joules
= 28,800 J
Cell Potential
(1) “The difference in potentials of the two half – cells of a cell known as electromotive force (emf) of the cell or cell potential.”
The potential difference of the two half – cells of a cell arises because of the flow of electrons from anode to cathode and flow of current from cathode to anode.

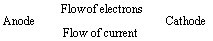
(2) The emf of the cell or cell potential can be calculated from the values of electrode potentials of two half – cells constituting the cell. The following three methods are in use
(i) When oxidation potential of anode and reduction potential of cathode are taken into consideration
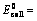 Oxidation potential of anode + Reduction potential of cathode
Oxidation potential of anode + Reduction potential of cathode 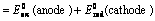
(ii) When reduction potentials of both electrodes are taken into consideration
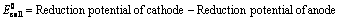
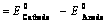
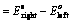
(iii) When oxidation potentials of both electrodes are taken into consideration
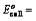 Oxidation potential of anode – Oxidation potential of cathode
Oxidation potential of anode – Oxidation potential of cathode 
Nernst Equation
Any change in the Gibbs free energy G directly correspond to changes in free energy for processes at constant temperature and pressure, change is the maximum non-expansion work obtainable under these conditions in a closed system; ΔG is negative for spontaneous process, positive for nonspontaneous process, and zero for processes at equilibrium.
It takes into consideration the values of the standard electrode potentials, temperature, activity and the reaction quotient for the calculation of cell potential. For any cell reaction, that occurs Gibbs free energy can be related to standard electrode potential as:
ΔG =-nFE
Where, n = number of electrons transferred in the reaction, ΔG= Gibbs free energy, E= cell potential F = Faradays constant (96,500 C/mol) and. Under standard conditions, the above equation can be written as,
ΔGo =-nFEo
According to the theory of thermodynamics, Gibbs free energy under general conditions can be related to Gibbs free energy under standard condition and the reaction quotient as:
ΔG=ΔGo + RT lnQ
Where, Q= reaction quotient, R= universal gas constant and T= temperature in Kelvin. Incorporating the value of ΔGo and ΔG, from the first two equations, we get the equation:
-nFE = -nFE0 + RT lnQ
E = E0 – (RT/nF) lnQ
By conversion of Natural log to log10, the above equation is called as the Nernst equation. Here, it shows the relation of the reaction quotient and the cell potential. Special cases of Nernst equation:
E = Eo − (2.303RT/nF) log10Q
At standard temperature, T= 298K:
E = Eo − (0.0592V/n) log10Q
At standard temperature T = 298 K, the 2.303RTF, term equals 0.0592 V.
Under Equilibrium Condition
As the redox reaction in the cell progresses, the concentration of reactants decreases while the concentration of products increases. This process goes on until equilibrium is achieved. At equilibrium, ΔG = 0. Hence, cell potential, E = 0. Thus, the Nernst equation can be modified to:
E0 – (2.303RT/nF) log10Keq = 0
E0 = (2.303RT/nF) log10Keq
Where, Keq = equilibrium constant and F= faradays constant. Therefore, the above equation gives us a relation between standard electrode potential of the cell where the reaction takes place and the equilibrium constant.
- Applications of Nernst Equation
One of the major applications of Nernst equation is in determining ion concentration
- It is also used to calculate the potential of an ion of charge “z” across a membrane. It is used in oxygen and the aquatic environment.
- It is also used in solubility products and potential-metric titrations.
- It is also used in pH measurements and to determine the Concentration Cell is an electrochemical cell.
Galvanic cell Voltaic cell is also known as electrochemical cell, these cells generate electrical energy using chemical reaction.
In a spontaneous energy is released, electrons move from one species to another in an oxidation -reduction reaction. The energy that is formed is split into two separate half reaction namely oxidation and reduction. A voltaic cell is formed with two containers and a wire, the reactions help the electrons move from one end to the other.
Principle of Galvanic (Voltaic) Cell
The cell consists of two half cells and salt bridge, and the electrical work is done by the Gibbs spontaneous energy. Of redox reactions. The cell that consists of Half cells further consist of a metallic electrode dipped in an electrolyte, the two cells are connected to a voltmeter and with the help of metallic wires is connected to an external switch, salt bridges are sometimes not required if both the electrodes are dipped in the same electrolyte.
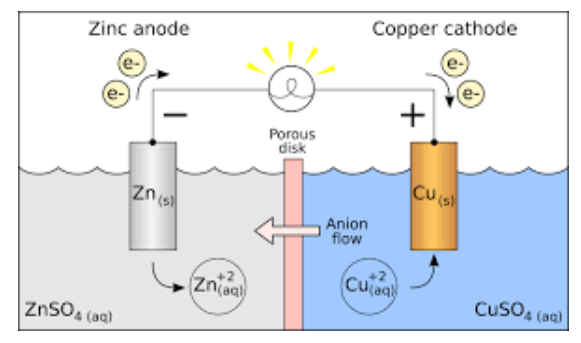
Galvanic Cell (Voltaic Cell) Diagram
It’s an activity series, that consists of a list of elements in a particular order according to their increasing potential values. The list of elements is based by measuring by measuring the potential of various electrodes versus standard hydrogen electrode (SHE).
In electrochemical series, the electrodes (metals and non-metals) in contact with their respective ions are arranged on the basis of the values of their oxidation potential or the potential of the elements is obtained by measuring the voltage, when the half cells are connected to the hydrogen electrode under standard conditions.
Primary cell: These cells or batteries are the ones that cannot be recharged after one use, after discharge they are usually discharged. These cells cannot be charged as the electrode reaction occurs only once and after usage over a period of time, they cannot be reused and become dead. Main disadvantage of a primary cell is that it can deliver current for short period of time only because, the quantity of oxidizing and reducing agent is limited. E.g.: dry cell.
Secondary cell: These cells or batteries are the one that can be electrically recharged after they are completely discharged. The recharging happens by passing current through the circuit in the opposite direction during discharge E.g. Smart phones
Fuel cell: Fuel cells are another means by which chemical energy can be converted into electrical energy. But energy can indefinitely be obtained from fuel cell as long as outside supply of fuel is maintained.