Unit - 7
Organic reactions and synthesis of a drug module
Reaction mechanisms describe the process how the material in a chemical reaction starts from the initial reactants and ends at the final products. One reaction that describes a reaction mechanism is the chemical reaction between nitrogen monoxide and oxygen to form nitrogen dioxide:
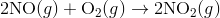
It seems as though this reaction occurs due to the collision between two NO molecules with one O 2 molecule. However, careful analysis of the reaction has detected the presence of N 2 O 2 during the reaction. A proposed mechanism for the reaction consists of two elementary steps:
Step 1: 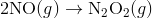
Step 2: 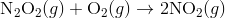
In the first step, two molecules of NO collide to form a molecule of N 2 O 2. However, in the second step, that molecule of N 2 O 2 collides with a molecule of O 2 to produce two molecules of NO 2. The overall chemical reaction is the sum of the two elementary steps:
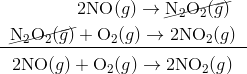
The N 2 O 2 molecule is not part of the overall reaction. It was first produced in the elementary step, the N 2 O 2 molecule then reacts in the second elementary step. An intermediate is a process which appears in the mechanism of a reaction, but not in the overall balanced equation. An intermediate is always formed in an early step in the mechanism and consumed in a later step.
These are the four "prototypical" organic chemistry reactions, though several others which can be categorized as one of these are generally referred to by other names. A glance at these reactions and these question for each would arise: what bonds are formed, and what bonds are broken.
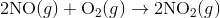
Fig. 1: Four general reactions in Organic Chemistry.
Which of the bonds are broken, and which bonds are formed? A rearrangement reaction is not demonstrated.
At least 80% of the reactions in organic chemistry fall into one of these four categories. The sooner the bond formation and breakage are recognized, a more clearer picture is visible. A fifth reaction is also discussed later in the chapter: rearrangement reactions.
Acid/Base Reactions
Another important category of organic reactions are straight-forward Brønsted–Lowry acid-base reactions.
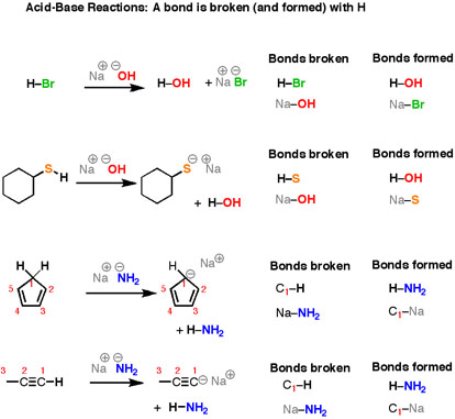
Fig.2: Acid/Base reactions
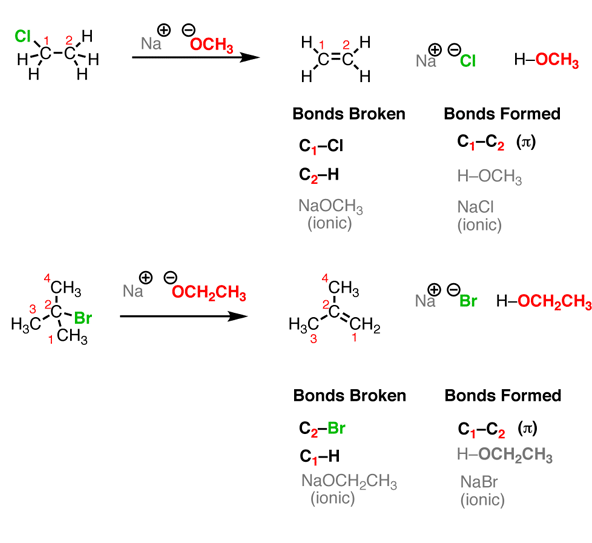
It can be noticed that the reactions look different on the surface – all those different structures! – but the basic plot of each reaction is the same. we are breaking an H-(atom) bond and forming an H-(atom) bond. At the same time, two “leftover” partners connect together to form a salt, composed of two oppositely-charged ions. Let’s look at that last reaction in more detail. Here, the breaking of a C-H bond and an (ionic) Na-NH2 bond, and forming an N-H bond as well as an (ionic) C-Na bond.
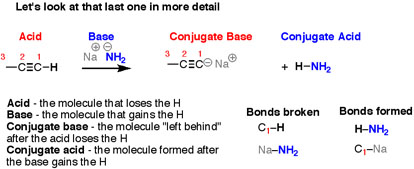
Fig. 3: There are four “actors” in this reaction – as there are in every acid-base reaction –
When, the reverse of the previous reaction is looked into carefully, the breaking of a bond to H and forming a bond to H happens, but after swapping everything, the breaking of N-H and C-Na takes place, and forming of N-Na and C-H occurs.
It’s still an acid-base reaction.
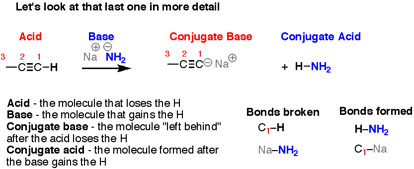
Fig.4: The four reactions that take place in this reaction, Acid-Base-Conjugate Base-Conjugate Acid.
However, experiment tells us that this reaction does not happen to any appreciable extent.
In this acid/base reaction, which are the bonds that are broken and which bonds are formed, if any?
(CH3−CH2)3N+HCl→(CH3−CH2)3NH++Cl−onumber (27.1.1) (27.1.1) (CH3−CH2)3N+HCl→(CH3−CH2)3NH++Cl−onumber
When an alkene (or alkyne) is taken and certain types of reagents are added to them, the resulting reaction is shown. if any bonds can be recognized, the bonds formed and broken should be recognised.
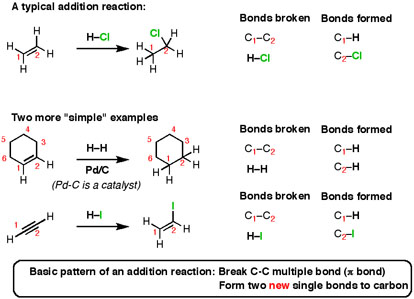
Fig.5: The reaction shows an Addition reaction, to an Alkene a suitable reagent is added, the C-C multiple bonds are broken and two new single carbon bonds are formed.
Here’s the basic pattern: break a C-C multiple bond (also called a π bond) and form two new single bonds (“σ-bonds” to carbon). This reaction is called an “addition reaction” and it’s a typical type of common reaction. Note that the reaction occurs only at the carbons that are a part of a multiple bond – nothing else on the molecule gets affected.
In this addition reaction, the question arises as to, which bonds are broken and which are formed, if any?
CH3−C(=O) −CH3−→−−−−H+/H2OLiAlH4CH3−CH(OH)−CH3onumber (27.1.2) (27.1.2) CH3−C(=O) −CH3→H+/H2OLiAlH4CH3−CH(OH)−CH3onumber
Note: This reaction could be described as a (nucleophilic) addition reaction or a reduction.
Substitution Reaction
Here are three examples of nucleophilic substitution reactions.
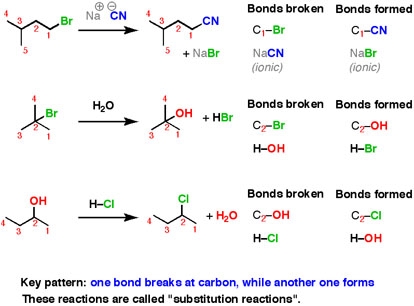
Fig.6: An example of nucleophilic substitution reactions.
In each case, we are breaking a bond at carbon, and forming a new bond at carbon. This is a very common pattern for reactions in organic chemistry, some interesting results are obtained from those experiments, most of the information we don’t get by thinking about what happens next and predict it.
In this substitution reaction, which bonds are broken and with are formed, if any?
CH3−C(=O) −OH+CH3−OH−→−−−−−− Δ and reflux H2SO4CH3−C(=O) −O−CH3+H2Oonumber (27.1.3) (27.1.3) CH3−C(=O) −OH+CH3−OH→ Δ and reflux H2SO4CH3−C(=O) −O−CH3+H2Oonumber
Note: This reaction would usually be called a condensation reaction.
Elimination Reactions
Let’s look at two simple cases. One important thing to keep in mind is that, although you see “Na OCH3” written here, the “Na+” (sodium) is not important for our purposes. It could alternatively be K+ (potassium) or Li+ (lithium). It’s just balancing the negative charge on the oxygen. When you take an alkyl halide and add a strong base (such as NaOCH3NaOCH3 or NaOCH2CH3NaOCH2CH3) a reaction occurs. See if you can recognize the bonds broken and formed.
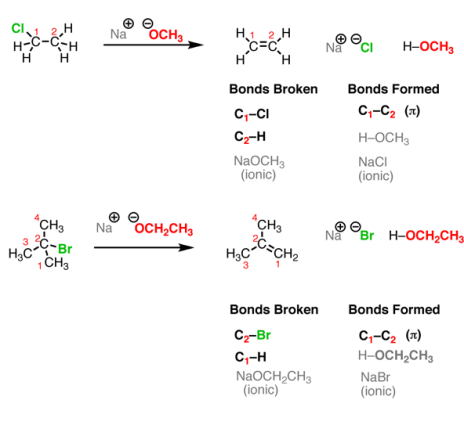
Fig.7: Elimination Reactions, these reactions are the reverse of addition reactions.
This reaction results in the formation of a new C-C double bond (π bond) and breaking of two single bonds to carbon (in such cases, one of them is H and the other is a halide such as Cl or Br). However, a new bond is formed between H and the O. So, in this case, this reaction incorporates a pattern we have already seen before – an acid-base reaction. Finally, a salt is formed in this reaction. But since the prime interest here is the organic product (that is, the one containing carbon), however, the salt by product is not written in some reaction schemes, but that doesn’t mean that it’s not present there.
NOTE
The three events in the elimination reaction of Fig (formation of C-C π bond, and breaking of two adjacent carbon sigma bonds) are the exactly opposite to the addition reactions discussed before.
Another example of an elimination reaction, is when 2-bromopropane is heated under reflux with a concentrated solution of sodium or potassium hydroxide in ethanol. Heating under reflux involves the heating with a condenser placed vertically in the flask to avoid loss of volatile liquids. The product formed from this reaction is Propene is and, because this is a gas, it passes through the condenser and is be collected.
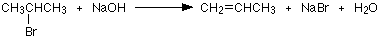

Everything else that is present (including anything formed in the alternative substitution reaction) will be trapped in the flask.
In this elimination reaction, which bonds are broken and with are formed, if any?
CH3−CH(OH)−CH3−→−−−−K2Cr2O7H2SO4CH3−C(=O) −CH3onumber (27.1.4) (27.1.4) CH3−CH(OH)−CH3→K2Cr2O7H2SO4CH3−C(=O) −CH3onumber
Note: This reaction can also be described an oxidation reaction.
Rearrangement reactions can accompany many of the reactions that were previously covered such as substitution, addition, and elimination reactions. These reactions are comparatively very rare. In fact, however when closely looked, sometimes it’s a fact that few rearrangements reaction has occurred and have been missed or gone unnoticed. Let’s look at a substitution reaction first.
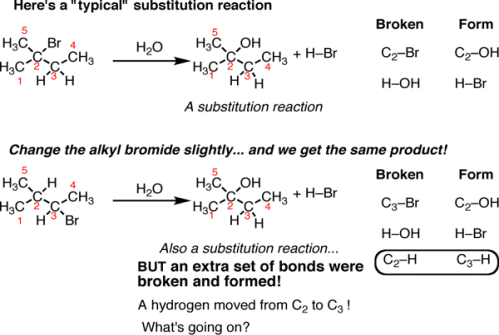
Fig.8: A typical substitution reaction, the C-Br bond is broken and a C-OH bond is formed.
On the top is a “typical” substitution reaction: we are taking an alkyl halide and adding water. The C-Br bond is broken and a C-OH bond is formed. If we look at the table on the right a typical pattern of substitution reactions is followed. However, if we change one thing about this alkyl halide – move the bromine to C-3 instead of C-2 – subsequently in this reaction a different product emerges. This is also a substitution reaction (where Br is replaced with OH) but it’s on a different carbon. when noticed closely, there are actually 3 bonds broken and 3 bonds formed. The C2-H bond broke and the C3-H bond formed.
In this Rearrangement reaction, which bonds are broken and with are formed, if any?
CH3−CH2−CH2−C(OH)=CH2→CH3−CH2−CH2−C(=O) −CH3onumber (27.1.5) (27.1.5) CH3−CH2−CH2−C(OH)=CH2→CH3−CH2−CH2−C(=O) −CH3onumber
Note: This reaction could also be described an oxidation reaction.
We find examples of oxidation-reduction or redox reactions almost every time we analyse the reactions used as sources of either heat or work. When natural gas burns, for example, an oxidation-reduction reaction occurs that releases more than 800 kJ/mol of energy.
CH4(g) + 2 O2(g)  CO2(g) + 2 H2O(g)
CO2(g) + 2 H2O(g)
The best example that happens within our bodies, is a sequence of oxidation-reduction reactions that occur to burn sugars, such as glucose (C6H12O6) and the fatty acids in the fats that we eat.
C6H12O6(aq) + 6 O2(g)  6 CO2(g) + 6 H2O(l)
6 CO2(g) + 6 H2O(l)
CH3(CH2)16CO2H(aq) + 26 O2(g)  18 CO2(g) + 18 H2O(l)
18 CO2(g) + 18 H2O(l)
These reactions can also be used as a source of energy, however, to find examples of oxidation-reduction reactions. Silver metal, for example, is oxidized when it comes in contact with trace quantities of H2S or SO2 in the atmosphere or foods, such as eggs, that are rich in sulfur compounds.
4 Ag(s) + 2 H2S(g) + O2(g)  2 Ag2S(s) + 2 H2O(g)
2 Ag2S(s) + 2 H2O(g)
surprisingly, the film of Ag2S that is formed collects on the metal surface and forms a protective coating that slows down further oxidation of the silver metal.
The given example of tarnishing of silver is just one of the reactions that occur in a broad class of oxidation-reduction reactions that come under the general heading of corrosion. Another example that occurs in a series of reactions when iron or steel rusts. When iron is heated, it reacts with oxygen to form a mixture of iron (II) and iron (III) oxides.
2 Fe(s) + O2(g)  2 FeO(s)
2 FeO(s)
2 Fe(s) + 3 O2(g) 2 Fe2O3(s)
Molten iron even reacts  with water to form an aqueous solution of Fe2+ ions and H2 gas.
with water to form an aqueous solution of Fe2+ ions and H2 gas.
Fe(l) + 2 H2O(l)  Fe2+(aq) + 2 OH-(aq) + H2(g)
Fe2+(aq) + 2 OH-(aq) + H2(g)
At room temperature, however, all three of these reactions are so slow they can be easily ignored.
Iron can only corrode at room temperature in the presence of both water and oxygen. In the sequence of this reaction, the iron gets oxidized to give a hydrated form of iron (II) oxide.
2 Fe(s) + O2(aq) + 2 H2O(l)  2 FeO H2O(s)
2 FeO H2O(s)
Because iron (II) oxide has the same empirical formula as Fe (OH)2, usually iron (II), is mistaken and also called as ferrous hydroxide. The FeO H2O formed in this reaction is further oxidized by O2 dissolved in water to give a hydrated form of iron (III), or ferric oxide.
4 FeO H2O(s) + O2(aq) + 2 H2O(l)  2 Fe2O3 3 H2O(s)
2 Fe2O3 3 H2O(s)
In a further complicated reaction, FeO H2O formed at the metal surface combines with Fe2O3 3 H2O to give a hydrated form of magnetic iron oxide (Fe3O4) shown in the reaction below.
FeO H2O(s) + Fe2O3  3 H2O(s) Fe3O4 n H2O(s)
3 H2O(s) Fe3O4 n H2O(s)
As these reactions only can occur in the presence of both water and oxygen, cars tend to rust easily wherever water collects. Furthermore, because the simplest way of preventing iron from rusting is to coat the metal surface so that it doesn't come in contact with water, cars were originally painted for only one reason  to slow down the formation of rust.
to slow down the formation of rust.
Diels-Alder Reaction (Nobel Prize in 1950)
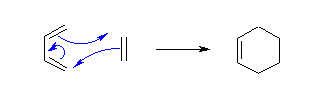
Due to the high degree of regio- and stereoselectivity (due to the concerted mechanism), the Diels-Alder reaction is a very powerful reaction and is popularly used in synthetic organic chemistry.
Dienes | 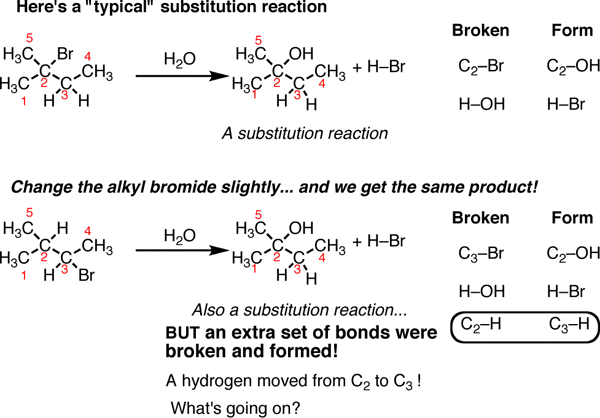 |
Dienophiles | 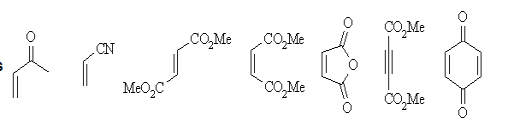 |
Stereoselectivity:
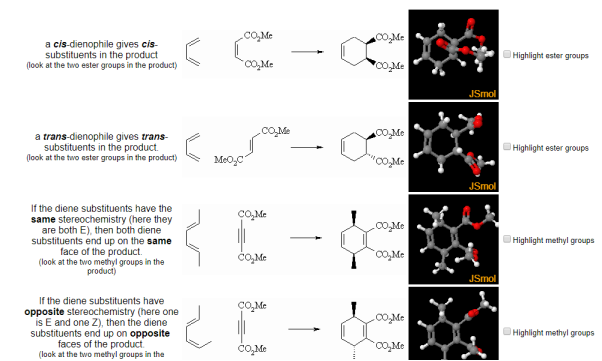
Fig.9: The picture shows the relative positions of dienophile and diene substituents.
Cyclic dienes can give stereoisomeric products depending on whether the dienophile lies under or away from the diene in the transition state. The endo product is usually the major product (due to kinetic control)
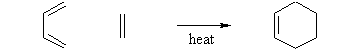
Fig.10: Cyclic dienes can give stereoisomeric products depending on whether the dienophile lies under or away from the diene in the transition state.
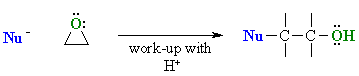
Reaction type: Nucleophilic Substitution
Summary
Depending on how a nucleophile reacts, there are two possible general scenarios:

Examples of such nucleophilic systems
are: RMgX, RLi, RC≡CM, LiAlH4, NaBH4

Examples of such nucleophilic systems are: H2O, ROH, R-NH2
Aspirin (acetylsalicylic acid) is a synthetic organic derived from salicylic acid. Salicylic acid is a natural ingredient that was used by the ancient Greeks and Native Americans, among others, it found in the bark of the willow tree and is used to counter fever and pain. However, it is known that salicylic acid is bitter and irritates the stomach.
Felix Hoffman a German chemist, is credited with being the first to synthesize aspirin in 1897. Hoffman's father had severe arthritis he used salicylic acid to relieve pain but could not tolerate salicylic acid. The name given for Hoffman's new compound was A-spirin. Apparently, this name comes from acetylation (A-), together with Spirin that is part of the name given for Meadow-sweet (Spiraea ulmaria), a plant rich in salicylates.
Friedrich Bayer, the employer of Hoffman, patented the name and began marketing the product in 1899. The product was a huge success and sales grew rapidly. Bayer's company, which he set up by himself, is reckoned to have been the first pharmaceutical company, and the production of aspirin is accepted to have laid the foundation of the modern pharmaceutical industry.
In this experiment aspirin (acetylsalicylic acid, C9H8O4) is synthesized and purified, and the percent yield is determined. The purity of the product formed is confirmed by measuring its melting point range and qualitative analysis.
The reaction that is used for the synthesis is shown below. In this reaction, an excess of acetic anhydride (C4H6O3) is added to a measured mass of salicylic acid (C7H6O3) in the presence of a catalyst, sulfuric acid (H2SO4). The mixture is heated to form the acetylsalicylic acid (C9H8O4) and acetic acid (C2H4O2). After the reaction takes place, water is added to destroy the excess acetic anhydride and cause the product to crystallize. The aspirin is then collected, purified by recrystallization, and its melting temperature measured.
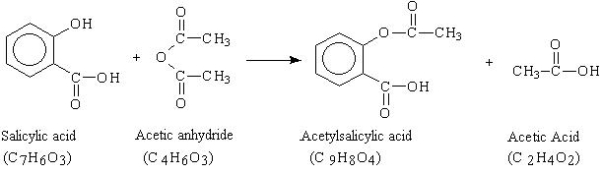
Fig.11: The equation shows the reaction of Salicylic acid with Acetic anhydride to form Aspirin.
References: