Module 04
Use of free energy in chemical Equilibria
4.1 Thermodynamic function: Energy, Entropy and Free energy
Classical thermodynamics has a formal structure which serves to organise knowledge and to establish relationships between well–defined quantities. It is in this context that extensive observations are taken to imply that energy is conserved. Therefore, the change in the internal energy ∆U of a closed system is given by
∆U = q − w ………………………(1)
Where q is the heat transferred into the system and w is the work done by the system. The historical sign convention is that heat added and work done by the system is positive, whereas heat given off and work done on the system are negative. Equation 1 may be written in differential form as
DU = dq – dw …………………….(2)
For the special case where the system does work against a constant atmospheric pressure, this becomes
DU = dq− P dV ………………………(3)
Where P is the pressure and V the volume. The specific heat capacity of a material is an indication of its ability to absorb or emit heat during a unit change in temperature. It is defined formally as dq/dT; since dq = dU + P dV , the specific heat capacity measured at constant volume is given by:
CV = µ ∂U ∂T ¶ V ………………(4)
It is convenient to define a new function H, the enthalpy of the system:
H = U + P V ………………….(5)
A change in enthalpy takes account of the heat absorbed at constant pressure, and the work done by the P ∆V term. The specific heat capacity measured at constant pressure is therefore given by:
CP = µ ∂H ∂T ¶ P
Entropy: Entropy is used to describe the behavior of a system in terms of thermodynamic properties such as temperature, pressure, entropy, and heat capacity. This thermodynamic description took into consideration the state of equilibrium of the systems.
Meanwhile, the statistical definition which was developed at a later stage focused on the thermodynamic properties which were defined in terms of the statistics of the molecular motions of a system. Entropy is a measure of the molecular disorder.
Free energy: In thermodynamics, energy-like property or state function of a system in thermodynamic equilibrium. Free energy has the dimensions of energy, and its value is determined by the state of the system and not by its history. Free energy is used to determine how systems change and how much work they can produce. It is expressed in two forms: the Helmholtz free energy F, sometimes called the work function, and the Gibbs free energy G. If U is the internal energy of a system, PV the pressure-volume product, and TS the temperature-entropy product (T being the temperature above absolute zero), then F = U − TS and G = U + PV − TS. The latter equation can also be written in the form G = H – TS, where H = U + PV is the enthalpy. Free energy is an extensive property, meaning that its magnitude depends on the amount of a substance in a given thermodynamic state.
4.2 Estimations of entropy and free energies
Thermodynamic principles can be employed to derive a relation between electrical energy and the maximum amount of work (Wmx).
The maximum amount of work obtainable from the cell is the product of charge flowing per mole and maximum potential difference E, through which the charge is transferred.
Wmax=−nFE
where,
n= number of moles electrons transferred
F= Faraday (96495 coulombs)
E=emf of the cell
According to thermodynamics,
Wmax=ΔG
∴ΔG=−nFE
when E is positive, ΔG is positive, ΔG will be negative and the cell reaction is spontaneous.
4.3 Free energy and emf
When a cell produces a current, the current can be used to do work - to run a motor, for instance. Thermodynamic principles can be employed to derive a relation between electrical energy and the maximum amount of work, Wmax, obtainable from the cell. The maximum amount of work obtainable from the cell is the product of charge flowing per mole and maximum potential difference, E, through which the charge is transferred.
Wmax = - n FE
Where,
n is the number of moles of electrons transferred and is equal to the valence of the ion participating in the cell reaction.
F stands for Faraday and is equal to 96,495 coulombs and E is the emf of the cell.
According to thermodynamics, the maximum work that can be derived from a chemical reaction is equal to the free energy (DG) for the reaction,
Wmax = DG
Therefore, from (1) and (2),
DG = - n FE
Thus only when E has a positive value, DG value will be negative and the cell reaction will be spontaneous and the e.m.f. Of the cell can be measured. Here E refers to the Ecell.
Thus, the electrical energy supplied by the cell is (nFE) equal to the free energy decrease (- DG) of the cell reaction occurring in the cell.
E.g.: Determine the standard emf of the cell and standard free energy change of the cell reaction.
Zn, Zn2+ || Ni2+, Ni.
The standard reduction potentials of Zn2+, Zn and Ni2+, Ni half cells are - 0.76 V and - 0.25 V respectively.
Eocell =EoR - EoL = - 0.25 - (- 0.76)
= + 0.51 V Eocell is + ve. \ DGo = - ve.
DGo = - n FEocell = 2 electrons
DGo = -2 * 96495 * 0.51
= -97460 Joules
= - 97.46
4.4 The Nernst equation and applications
This equation is named after the name of scientist who discovered is Walther Nernst. Nernst equation plays a major role in relating the Reduction Potential with the electrode potential, temperature of the chemicals which are undergoing the oxidation or reduction.(Reduction Potential is used to measure the tendency of the chemical species to acquire or loose electron to an electrode.)
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation ………..(i)
 G=-nFE ………..(ii)
G=-nFE ………..(ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE* ………….(iii)
G*=-nFE* ………….(iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ ………….(iv)
G= G*+RT lnQ ………….(iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ …………….(v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6) ……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q …….(vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q ……..(viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K …….(ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Applications:
1- This is used in the solubility product and potentio-metric titration.
2- It is used to calculate the potential of ion charge.
It is used in oxygen and aquatic environment.
4.5 Acid base, oxidation reduction and solubility equilibria
Acid-Base
The organic compound that possesses acidic properties is called as an organic acid. E.g.: carboxylic acid, whose acidity is associated with their carboxyl group –COOH. The relative stability of the conjugate base of the acid determines its acidity.
The organic compound that possesses basic properties is called as organic bases. Organic bases act as the protons acceptors. Organic bases usually contain the nitrogen atoms that can be easily protonated. E.g.: Amines that have a lone pair of electron on the nitrogen atom and hence it can act as the proton acceptor.
Bond Strength:
The stronger the A–H or B–H+ bond, the less likely the bond is to break to form H+H+ ions and thus the less acidic the substance. This effect can be illustrated using the hydrogen halides:
Acid Strength | HF | HCl | HBr | HI |
H-X Bond Energy | 570 | 432 | 366 | 298 |
PKa | 3.20 | -6.1 | -8.9 | -9.3 |
The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases.
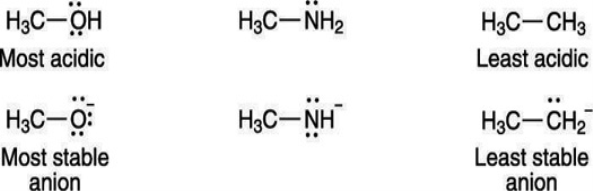
A negative charge prefers to rest on electronegative elements. Therefore, a negative charge is more stable on oxygen than it is on nitrogen similarly, a negative charge is more stable on nitrogen than it is on carbon. For that reason, alcohols (R—OH) are more acidic than amines (R—NH2), which in turn are more acidic than alkanes (R—CH3), as shown here:
Electro negativity affects acid strength:
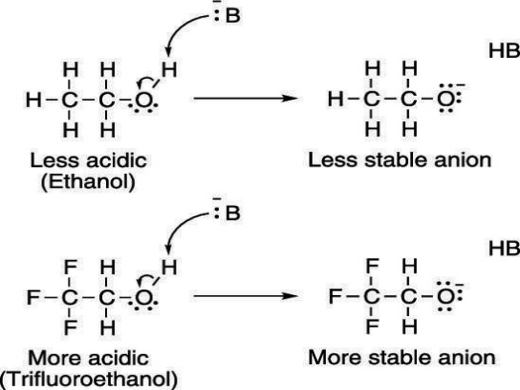
Electron-withdrawing groups on an acid also stabilize the conjugate base anion by allowing some of the charge on the anion to delocalize to other parts of the molecule. For example, trifluoroethanol (shown here) is more acidic than ethanol.
Bronsted-Lowry acid: The Bronsted-Lowry theory describes acid-base interactions in terms of proton transfer between chemical species. A Bronsted-Lowry acid is any species that can donate a proton, H+ and a base is any species that can accept a proton. In terms of chemical structure, this means that any Bronsted-Lowry acid must contain a hydrogen that can dissociate as H+. In order to accept a proton, a Bronsted-Lowry base must have at least one lone pair of electrons to form a new bond with a proton. Any species that is capable of donating a proton –H+ while the species that is capable of accepting a protons that requires a lone pair of electron to bond to the H+. E.g.: Water; as it is amphoteric that means it can act as both Bronsted-Lowry acid and a Bronsted-Lowry base. The ionization of weak acids and bases is partial while it is complete in strong acids and bases. The conjugate base of a Bronsted-Lowry acid is the species formed after an acid donates a proton. The conjugate acid of a Bronsted-Lowry base is the species formed after a base accepts a proton. The two species in a conjugate acid-base pair have the same molecular formula except the acid has an extra H+ ion compared to the conjugate base.
Oxidation-Reduction:
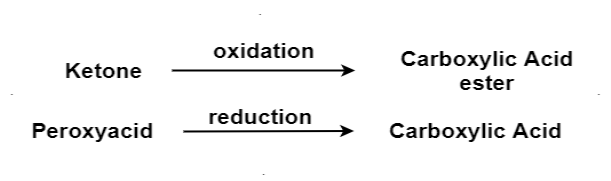
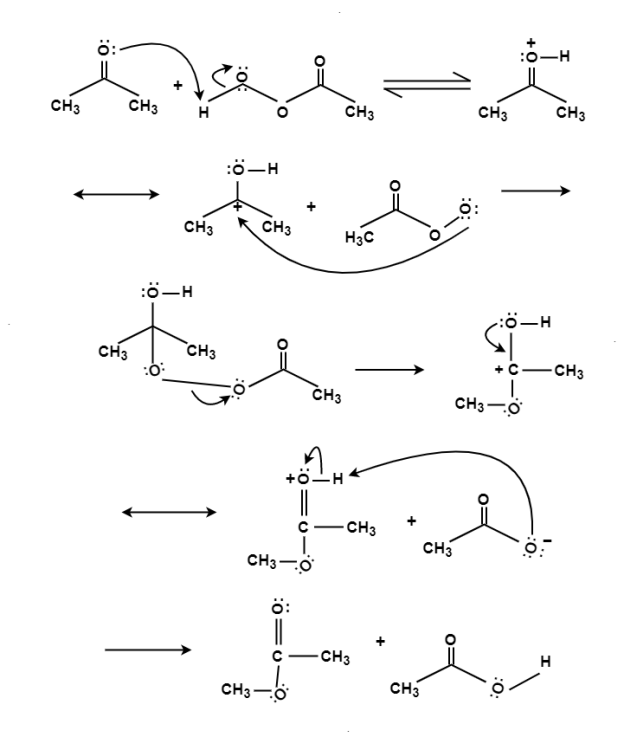
The oxidation of ketone to a carboxylic acid ester by the help of peroxy acid as the oxidizing agent.
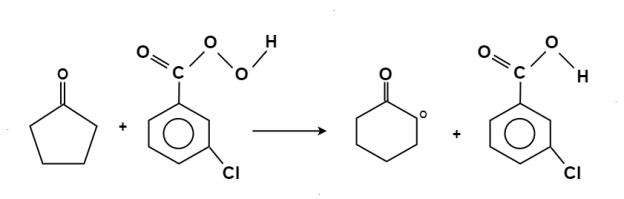
Mechanism:
Reduction (Clemmensen reduction, Wolff‐Kishner reduction):
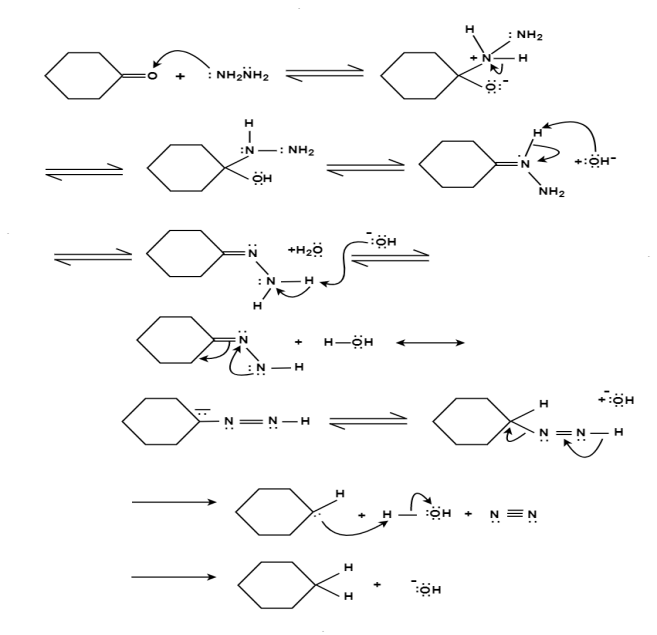
The aldehydes and ketones react with hydrazine in basic medium, which reduces the aldehyde or the ketone to a hydrocarbon, is called Wolff-Kishner reduction.
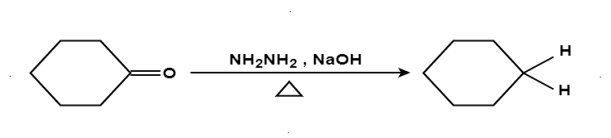
Mechanism:
4.6 Water chemistry
Ion exchange or de ionization or de mineralization ion exchange resins are insoluble cross linked long chain organic polymers with a microporous structure and the functional group attached to the chains are responsible for the ion exchange properties resins contaning acidic function groups are capable of exchaning their anions with other anions which comes in their contact the ion exchange resins may be classified as :-
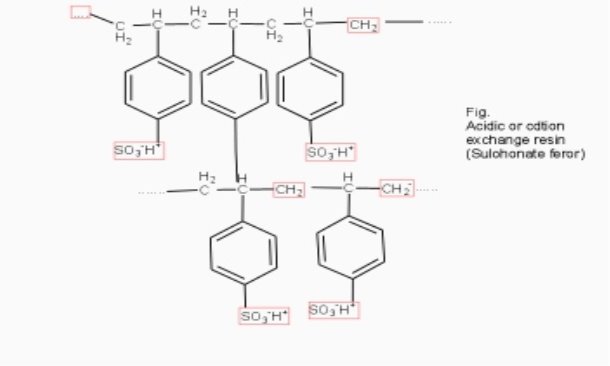
Cation exchange resins ( RH+) :-
They are mainly styrene divinly benzene co-polymers which on sulphonation or carbonoxylation become capable to exchange their hydrogen ions with the cations in the water.
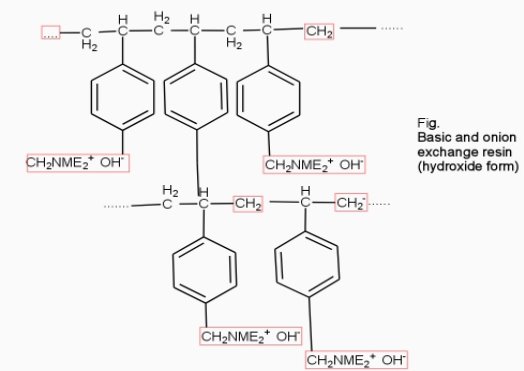
- Anion exchange resins :-
They are styrene – divinly benzene or amine fermaldehyde which contain amino or quaternary ammonium or4 quaternary phosphonium or tertiary sulphonium group as an integral part of the resin matrix.these after treatment with dilute NaOH solution become capable to exchange their OH- anions with anions in water .
Processes :-
The hard water is passed first through cation exchange column which removes all the cations like Ca²+ , Mg² + e.t.c . From it and equivalent amount of H+ ions are released from this column Water
2RH+ + Ca².+ ---------------------- R2Ca2+ + 2H
2RH+ Mg2+ --------------------------- R2Mg2+ 2H+
After cation exchange column the hard water passed through anion like cl- . Present in the water and equivalent amount of OH- ions are released from this column to water,
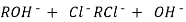
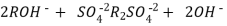
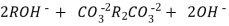
H+ And OH – ions ( released from cation exchange and anion exchange columns respectively ) get combined to produce water molecule.
H+ + OH -  H2O
H2O
Thus,
The water coming out form the exchanger is free from cations as well as an ions . Ion free water is known as de mineralizes water.
Regeneration :-
When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively . Are lost they are then said to be exhausted.
The exhausted cation exchange column is regenerated by passing a solution of dil.HclH2SO4. The regeneration can be represented as
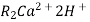

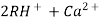 ( washing )
( washing )
The column is washed with deionized water and washing ( which contains Ca2+ and Cl2- ions ) is passed to sink or drain.
The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH.
The column is washed with de-ionized water and washing (which contains NA+ and 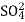 ions ) is pass to sink or drain.
ions ) is pass to sink or drain.
The regenerated ion exchange ion exchange resins are then used again.
4.7 Corrosion
The destruction of metal by chemical or electrochemical attack of environment this process starting at the surface of metal known as corrosion. Corrosion is the two step process that requires three things i.e., a metallic surface, an electrolyte and oxygen. In the process of corrosion a metal atom at surface dissolve into an aqueous solution leaving the metal with excess negatively charged ions. These resultant ions are removed by a suitable electron acceptor. Corrosion can be thought of as the spontaneous return of metals to their ores through the process of oxidation.
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
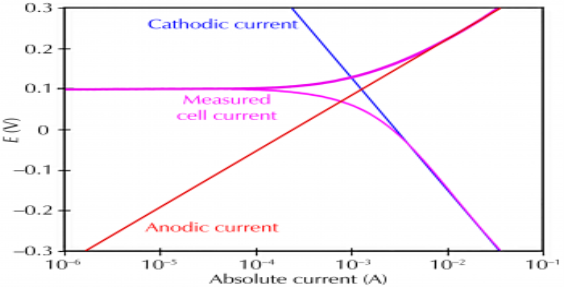
Corrosion process showing the anodic and cathodic component of current
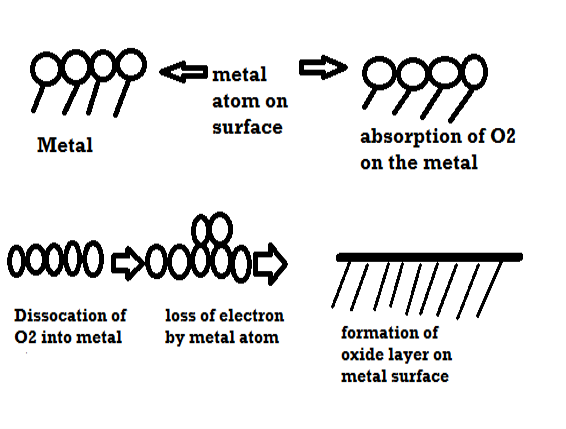
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
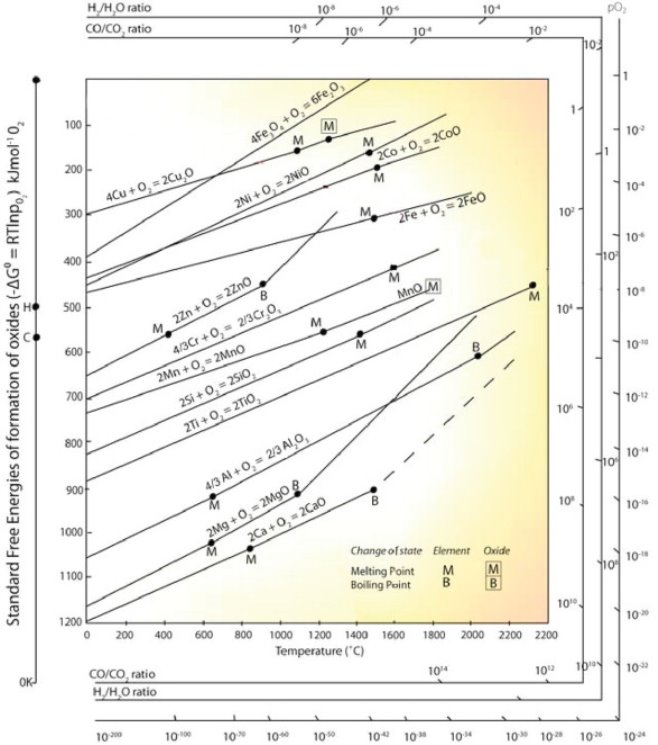
4.8 Use of free energy considerations in metallurgy through Ellingham diagram
Ellingham diagram is the graph which shows the temperature dependence of the stability of compounds. It is used to evaluate the ease of reduction of metal oxides and sulfides.
The salient features of Ellingham diagram for oxides are:
1) The straight lines represented by the function T in the given graph represent the relative stability of oxides.
2) It is possible to visualize directly the affinities of metals for oxygen in their standard conditions by observing the relative positions of the lines in the given graph.
3) It is possible to identify that there are easily reducible oxides of Au, Ag, Hg.