Unit - 15
Repairs & Rehabilitation of Structures
Corrosion is the deterioration or destruction of metals and alloys in the presence of an environment by chemical or electrochemical means. In simple terminology, corrosion processes involve reaction of metals with environmental species.
Mechanism of Corrosion:
The corrosion process that takes place in concrete is electrochemical in nature very similar to a battery. The mechanism of corrosion involves four basic elements
Anode: Site where metal atoms lose electrons i.e., where corrosion is initiated.
Cathode: Site where electrons flow to and combine with other metallic and non-metallic ion.
Electrolyte: A medium capable of conducting electric current by ionic current flow.
Metallic path: Connection between the anode and cathode that completes the circuit.
Corrosion is an irreversible interfacial reaction of a material (metal, ceramic, polymer) with its environment which results in its consumption or dissolution into the material of a component of the environment. Often, but not necessarily, corrosion results in effects detrimental to the usage of the material considered. Exclusively physical or mechanical processes such as melting and evaporation, abrasion or mechanical fracture are not included in the term corrosion” With the knowledge of the role of various microorganisms present in soil and water bodies, the definition for corrosion need be further widened to include microbially-influenced factors.
Corrosion can be classified in different ways, such as
Dry corrosion occurs in the absence of aqueous environment, usually in the presence of gases and vapours, mainly at high temperatures. Electrochemical nature of corrosion can be understood by examining zinc dissolution in dilute hydrochloric acid.
Zn + 2HCl = ZnCl2 + H2
Anodic reaction is Zn = Zn++ + 2e with the reduction of 2H+ + 2e = H2 at cathodic areas on the surface of zinc metal.
There are two half reactions constituting the net cell reaction. Environmental effects such as those of presence of oxygen and other oxidizers, changes in flow rates (velocity), temperature, reactant concentrations and pH would influence rates of anodic and cathodic reactions.
Even though the fundamental mechanism of corrosion involves creation or existence of corrosion cells, there are several types or forms of corrosion that can occur. It should however be borne in mind that for corrosion to occur, there is no need for discrete (physically independent) anodes and cathodes. Innumerable micro level anodic and cathodic areas can be generated at the same (single) surface on which anodic (corrosion) and cathodic (reduction) reactions occur. Each form of corrosion has a specific arrangement of anodes and cathodes and specific patterns and locations depending on the type can exist.
The most important types are
(a) UNIFORM CORROSION
Corrosion is an electrochemical process in which a metal reacts with its environment to form an oxide or other compound. The cell which causes this process has three essential constituents: an anode, a cathode and an electrically conducting solution. Simply, the anode is the site at which the metal is corroded; the electrolyte solution is the corrosion medium; and the cathode forms the other electrode of the cell and is not consumed in the corrosion process. At the anode the corroding metal passes into solution as positively-charged ions, releasing electrons which participate in the cathodic process. Thus, the rate of corrosion is equivalent to the electron current flowing between anode and cathode of the corrosion cell.
The distribution of anodic and cathodic areas is one of the most important factors determining the type of corrosion which occurs, but in the simplest case the corrosion cells are very small and numerous, and distributed in a random manner over the surface of the metal, and the effect is more or less uniform attack on the surface. The overall rate of the process under these circumstances may depend on a number of factors but the important criterion is the balance between rates of anodic and cathodic reactions required to maintain electro neutrality, and the overall rate of corrosion is controlled by the rate of whichever reaction occurs least readily. Although many metals display active corrosion behaviour when immersed in a corrosive solution, others including many engineering alloys carry a stable film of solid corrosion product, frequently an oxide.
If this film has good cohesive strength and adhesion to the metal, and low ion conductivity, the rate of metal dissolution is limited to the rate at which metal ions can pass through the film, often a factor of 103 – 106 times less than the rate of corrosion of unfilmed metals. This is the phenomenon of passivity, exhibited by stainless steels, nickel alloys, titanium and many similar materials and responsible for their utility as engineering materials in corrosive environments.
Although uniform corrosion is responsible for the greatest destruction of structural materials, it is not technically a major cause for concern. The rate of attack is measurable by relatively simple tests and the lifetime of plants and components can be predicted with confidence. It is the more complex forms of corrosion which present problems, in that they provide the means for localisation and acceleration of the attack, making possible plant failure with only little total metal loss, frequently through microscopic pits or cracks which may not be readily visible until failure has actually occurred.
(b) GALVANIC (BIMETALLIC) CORROSION
When two dissimilar metals are immersed in a conducting solution, they usually develop different corrosion potentials. If the metals are in contact this potential difference provides the driving force for increased corrosion, the less noble of the two metals corroding more rapidly, while the more noble corrodes less.
An electrochemical series based on the standard thermodynamic data for the metals is frequently used as a basis for ranking metals under these conditions, but a more practical means of ranking is a galvanic series determined experimentally for particular corrosion elements.
Dissimilar metal contacts provide the first example of situations in which the anodic and cathodic areas of a corrosion couple can be separated and therefore the rates of the two reactions may be substantially different, while electro
Neutrality is still maintained. Although tables of galvanic behaviour will show which alloy of a galvanic couple will become anodic and which cathodic, they give no indication of the rate of corrosion to be expected of the anodic material. For example, many active/passive materials which behave well in sea water and would be expected to be cathodic in many couples are in fact not very good cathodes and readily become polarised. Under these conditions acceleration of attack of the baser member of the couple is often less than might be expected. The prediction of rates is thus quite complex, and there is much qualitative tabular material available to provide assistance
Base Magnesium
Zinc
Aluminium (commercial)
Cadmium
Duralumin (Al with 4½% Cu)
Mild steel
Cast iron
Stainless steel (Type 430; 18% Cr) ACTIVE
Stainless steel (Type 304; 18% Cr 10% Ni) ACTIVE
Lead-tin solders
Lead
Tin
Nickel
Brasses
Copper
Bronze
Monel
Silver solders (70% Ag 30% Cu)
Nickel PASSIVE
Stainless steel (Type 430) PASSIVE
Stainless steel (Type 304) PASSIVE
Silver
Titanium
Graphite (Carbon) (non-metal)
Gold
Noble Platinum
Two further factors are of major importance, an environment/distance effect and the area ratio effect. The former is straightforward, simply that galvanic effects are reduced in proportion to the distance from the cathodic region and to the resistivity of the environment – the enhancement of attack at anodic regions is much reduced in poorly conducting solutions, where the resistance of the corrosion cell plays a major part in controlling the corrosion current. The area ratio effect is the key to the practical significance of galvanic coupling, for if a large cathodic area is coupled to a small anodic area (and ratios of hundreds and sometimes thousands of times occur in practice), then the rate of attack of the anodic material may become very high indeed. Conversely, large anodes coupled to small cathodes may not give problems even with unfavourable pairs of materials.
(c) CREVICE CORROSION
It is quite possible for corrosion to become localised on a single metal if environmental conditions are able to develop non-uniformly over the surface. The existence of crevices, either as a result of design or by development of deposits etc, can lead to occluded regions in which oxygen, the usual cathodic reactant, cannot be replenished. Whereas both anodic and cathodic reactions once occurred uniformly over the surface, after a short period the only reaction remaining in the crevice may be the anodic reaction, balanced by cathodic action outside the crevice. The resulting crevice corrosion is most serious in active/passive materials where a sequence of events may follow leading to significant and irreversible changes in the environment within the crevice.
(d) PITTING CORROSION
Pitting corrosion is another form of extremely localised attack by which plant can be seriously damaged while the overall metal loss is negligible. It occurs predominantly as a result of the action of chloride ions, although bromides, hypochlorites and a few other species are occasionally responsible, and it is particularly insidious because it frequently occurs in environments which are close to providing complete protection for the alloy except for occasional fluctuations in conditions. Again, the phenomenon is most serious in alloys which can cause major changes of the environment within the pit, when an autocatalytic increase in corrosion rate can occur.
(e) INTERGRANULAR ATTACK
Grain boundaries are somewhat more reactive than the matrix of an alloy, but since the difference in reactivity is slight, grain boundary effects are usually of little consequence. However, there are a few alloy systems in which very severe attack can occur at grain boundaries, usually as a result of improper heat treatment. Austenitic stainless steels may become sensitised to intergranular attack if they are heated in the range 500-800 °C. Chromium carbide is precipitated at grain boundaries, and by removing large amounts of chromium is precipitated at grain boundaries, and by removing large amounts of chromium from solid solution renders the zone adjacent to the boundary particularly susceptible to attack. This effect may be found in the heat-affected zone adjacent to welds, if too great a heat input has held the temperature of the zone in the sensitising range for a sufficient period. The problem is avoided either by correct heat treatment or more commonly by lowering the carbon level of the steel below 0.03 per cent or stabilising the carbon by the addition of strong carbide-formers such as titanium or niobium so that the carbon is no longer available for chromium carbide precipitation. High-strength aluminium alloys are also susceptible to intergranular corrosion if the heat treatment has been such as to cause the precipitation of the intermetallic phases, on which the alloys depend for their mechanical strength, in the grain boundary regions. The effect can again be avoided by proper alloy selection and careful heat treatment.
(f) STRESS CORROSION AND RELATED TOPICS
Stress corrosion is arguably the most serious of a number of phenomena involving the combination of mechanical factors and corrosive environments. The term refers to the apparently brittle (low macroscopic ductility) failure of a number of alloys in very specific environments, under tensile stresses which may be significantly lower than the tensile strength of the material and frequently lower than the yield strength. During stress-corrosion cracking the alloy is virtually unattacked over most of its surface, while fine cracks grow through it. Since it is extremely difficult to detect stress corrosion whilst it is occurring, the failure which occur are frequently unexpected and sometimes catastrophic. The fracture paths may be intergranular or transgranular depending on the system, and although they may branch extensively, they run generally normal to the tensile component of the stress. After an induction period, which may be very long, the crack growth rates can become extremely high. The variables involved include environment composition and temperature, metal composition and structure, and stress. A number of mechanisms have been proposed, including pre-existing chemically- or physically-active paths, yield assisted enhanced corrosion rates and film rupture mechanisms, and each probably has its validity in different alloy systems.
(g) HYDROGEN EMBRITTLEMENT
It has become common for engineers to include hydrogen embrittlement under the blanket term stress-corrosion cracking, although the two processes are mechanistically quite distinct. Hydrogen embrittlement is a mode of low ductility fracture which may be induced in ferritic and martensitic steels at high strength levels and in titanium alloys by the introduction of hydrogen. Although it is relatively simple to demonstrate the distinction between hydrogen embrittlement and true stress corrosion in suitable laboratory tests it is by no means easy to separate the mechanisms in practical failure situations with high-strength materials. Thus, for example much of the stress-corrosion cracking reported in alloy steels in hydrogen sulphide or cyanide environments is undoubtedly due to the effects of hydrogen rather than to anodic processes. The hydrogen may arise from a variety of sources including electroplating and welding, but the problem is of greater concern when the hydrogen is generated by corrosion. Not only is this continuously available while the alloy is exposed, but for sufficiently susceptible alloys the only environmental requirement is the presence of water in some form.
(h) CORROSION FATIGUE
Corrosion fatigue may be described as a reduction in fatigue resistance due to the action of a corrosive environment. Thus, it is determined by the loss in mechanical properties rather than by a particular mechanism or failure appearance. In fact, the fatigue performance of most metals varies from in vacuo testing conditions through ambient laboratory conditions to obviously corrosive situations, but a measurable difference may be expected between ambient laboratory fatigue data and data obtained in corrosive environments. Some authors would consider corrosion fatigue to be a special case of stress corrosion cracking, but the mode of failure is usually closer to fatigue than to stress corrosion and the phenomenon occurs over a wider range of materials and environments.
Distress: Distress can be thought of as the symptoms indicating that the defects are present.
Types of Distress
Blow holes- sometimes also bug-holes, are individual rounded or irregular cavities that are formed against the formwork and become visible on stripping of the formwork.
Cold joints- These are created when new concrete is poured against the concrete that has just hardened.
Honey Combing- It refers to voids caused by the mortar not filling the spaces between the coarse aggregate particles.
Crazing- It is the network of fine random cracks that are formed due to the shrinkage of the layer relative to the base concrete. It does not pose any structural or Serviceability problem.
Pop-outs- Rough conical depressions in the concrete surface caused by the expansion of the deleterious aggregate particles near the surface or expansion due to freezing are called pop-outs.
Disintegration two terms generally used to mark this they are Scaling- Localized flaking or peeling away of the near surface portion of the hardened concrete due to freeze thaw, Dusting White powdery formation on the surface of hardened concrete that receives excessive traffic.
Cracking- Cracking in concrete is inherent. Type of structure and nature of cracking is the major concern. Cracks in the concrete does not always mean that the structure is unusable.
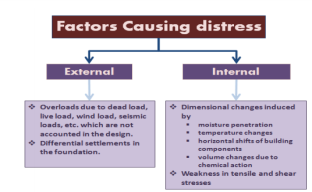
Structural Cracks- Structural cracks are those that may occur due to deficient designs, overloading, abnormal vibrations, use of inferior quality materials, foundation placed on uncompacted/loose soils, adoption of improper construction practices, poor workmanship, etc.
Non-Structural Cracks- These cracks occur due to the internally induced stresses in building material or due to the temperature induced movement of the materials. These cracks mar the appearance of the structure and at time may give a feeling of instability.
Settlement Cracks

Spalling- It is development of the fragments usually in the shape of the flakes, due to corrosion of steel or freeze thaw effects.

Stain- It is white powdery surface which may be caused by alkali-aggregate reactions. The stain may sometimes be colored due to corrosion of reinforcement.
Erosion- It could be due to abrasion, erosion which is marked by smooth, well-worn abraded surface of concrete, while in cavitation- erosion concrete appears to be very rough and pitted.
Corrosion- Rusting of steel in concrete, this result in cracking or spalling.
Deflection- It is the bending or sagging of the reinforced concrete structural elements like beams, slabs, columns, etc., which can be due to overloading, corrosion, or by creep in concrete.
Scaling of Concrete
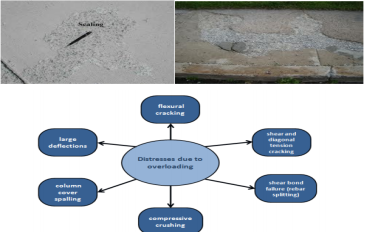
Rehabilitations
The success of repair activity depends on the identification of the root cause of the deterioration of the concrete structures. The repairs can be done for the improvement of strength and durability, thus extending the life of the structure, is not difficult to achieve.
It is the processes of restoring the structure to service level, once it had and now lost, strengthening consists in endowing the structure with a service level, higher than that initially planned by modifying the structure not necessarily damaged area.
The following steps are generally used in the rehabilitation of distressed concrete structure:
Applications:
NDT stands for Non-Destructive Testing. It refers to an array of inspection methods that allow inspectors to evaluate and collect data about a material, system, or component without permanently altering it.
NDT may also be called:
NDT CODES AND STANDARDS
NDT techniques can be used for all kinds of inspections. But some of the most important types of NDT inspections are of assets like boilers and pressure vessels, which could be incredibly dangerous if not properly maintained.
Because proper maintenance of these assets is so important for the safety of those working nearby (or even at a distance, when it comes to nuclear power plants), most countries have laws requiring companies to adhere to specific inspection codes and standards when conducting inspections.
These standards and codes typically require inspections to be conducted periodically following specific guidelines. For the most assets that present the greatest risk, these inspections must be both conducted by a certified inspector and approved by a certified witness working for a formal inspection body.
Here are the most commonly followed organizations in the world for creating NDT standards and codes:
WHY USE NDT?
Here are the top reasons NDT is used by so many companies throughout the world:
Non-destructive testing is the life blood of a well-run facility. NDT techniques and repeatable results depend on highly trained technicians with experience and integrity. Industrial NDT methods and interpretation of results are performed by certified professionals. Not only does the technician need to be certified in a specific NDT method, but they also need to know how to operate the equipment being used to gather data. Understanding equipment capabilities and limitations is the difference between making an accept or reject determination.
8 Most Common NDT Methods
There are several techniques used in NDT for the collection of various types of data, each requiring its own kind of tools, training, and preparation.
Some of these techniques might allow for a complete volumetric inspection of an object, while others only allow for a surface inspection. In a similar way, some NDT methods will have varying degrees of success depending on the type of material they’re used on, and some techniques—such as Magnetic Particle NDT, for example—will only work on specific materials (i.e., those that can be magnetized).
Here are the eight most commonly used NDT techniques:
1. VISUAL TESTING (VT)
Definition: Visual Non-Destructive Testing is the act of collecting visual data on the status of a material. Visual Testing is the most basic way to examine a material or object without altering it in any way.
How to conduct Visual Testing
Visual Testing can be done with the naked eye, by inspectors visually reviewing a material or asset. For indoor Visual Testing, inspectors use flashlights to add depth to the object being examined. Visual Testing can also be done with an RVI (Remote Visual Inspection) tool, like a camera. To get the camera in place, NDT inspectors may use a robot or drone, or may simply hang it from a rope.
2. ULTRASONIC TESTING (UT)
Definition: Ultrasonic Non-Destructive Testing is the process of transmitting high-frequency sound waves into a material in order to identify changes in the material’s properties.
How to conduct Ultrasonic Testing
In general, Ultrasonic Testing uses sound waves to detect defects or imperfections on the surface of a material created.
One of the most common Ultrasonic Testing methods is the pulse echo. With this technique, inspectors introduce sounds into a material and measure the echo’s (or sound reflections) produced by imperfections on the surface of the material as they are returned to a receiver.
Here are some other types of Ultrasonic Testing:
3. RADIOGRAPHY TESTING (RT)
Definition: Radiography Non-Destructive Testing is the act of using gamma- or X-radiation on materials to identify imperfections.
How to conduct Radiography NDT Testing
Radiography Testing directs radiation from a radioactive isotope or an X-ray generator through the material being tested and onto a film or some other kind of detector. The readings from the detector create a shadowgraph, which reveals the underlying aspects of the inspected material.
Radiography Testing can uncover aspects of a material that can be hard to detect with the naked eye, such as alterations to its density.
4. EDDY CURRENT (ELECTROMAGNETIC) TESTING (ET)
Definition: Eddy Current Non-Destructive Testing is a type of electromagnetic testing that uses measurements of the strength of electrical currents (also called eddy currents) in a magnetic field surrounding a material in order to make determinations about the material, which may include the locations of defects.
How to conduct Eddy Current Testing
To conduct Eddy Current Testing, inspectors examine the flow of eddy currents in the magnetic field surrounding a conductive material to identify interruptions caused by defects or imperfections in the material.
5. MAGNETIC PARTICLE TESTING (MT)
Definition: Magnetic Particle Non-Destructive Testing is the act of identifying imperfections in a material by examining disruptions in the flow of the magnetic field within the material.
How to conduct Magnetic Particle Testing
To use Magnetic Particle Testing, inspectors first induce a magnetic field in a material that is highly susceptible to magnetization. After inducing the magnetic field, the surface of the material is then covered with iron particles, which reveal disruptions in the flow of the magnetic field. These disruptions create visual indicators for the locations of imperfections within the material.
6. ACOUSTIC EMISSION TESTING (AE)
Definition: Acoustic Emission Non-Destructive Testing is the act of using acoustic emissions to identify possible defects and imperfections in a material.
How to conduct Acoustic Emission Testing
Inspectors conducting Acoustic Emission Tests are examining materials for bursts of acoustic energy, also called acoustic emissions, which are caused by defects in the material. Intensity, location, and arrival time can be examined to reveal information about possible defects within the material.
7. LIQUID PENETRANT TESTING (PT)
Definition: Liquid Penetrant Non-Destructive Testing refers to the process of using a liquid to coat a material and then looking for breaks in the liquid to identify imperfections in the material.
How to conduct Penetrant Testing
Inspectors conducting a Penetrant Test will first coat the material being tested with a solution that contains a visible or fluorescent dye. Inspectors then remove any extra solution from the material’s surface while leaving the solution in defects that “break” the material’s surface. After this, inspectors use a developer to draw the solution out of the defects, then use ultraviolet light to reveal imperfections (for fluorescent dyes). For regular dyes, the color shows in the contrast between the penetrant and the developer.
8. LEAK TESTING (LT)
Definition: Leak Non-Destructive Testing refers to the process of studying leaks in a vessel or structure in order to identify defects in it.
How to conduct Leak Testing
Inspectors can detect leaks within a vessel using measurements taken with a pressure gauge, soap-bubble tests, or electronic listening devices, among others.
Non-Destructive Testing Uses
Depending on how broadly you define NDT you could say that it’s used in almost every industry in the world, since visual inspections (whether formalized or casual) take place in almost every workplace in some form or other.
That being said, there are specific industries that require NDT and have formalized processes for its use, as codified by those organizations we listed above like API and ASME.
These industries include:
Composites are increasingly being used to repair structures built with other materials. Target applications include bridge beams, bridge decks, parking garages and pipelines, including underground systems. A number of specialty contractors, such as Structural use onsite-impregnated composite fabrics and procured reinforcement strips to add load capacity and strength to concrete floors and beams.
Steel-reinforced concrete can be damaged by seismic events and is commonly compromised by temperature-induced contraction and expansion. This results in cracks, which permit moisture invasion, results in corrosion and spalling (expansion) of the steel rebar. That, in turn, aggravates cracking and leads, eventually, to concrete disintegration followed by structural failure. Composites are increasingly used to repair such structures at significantly less cost than new construction, and also to rehabilitate structures that must bear increasing loads beyond the structure’s rated capacity.
According to Jay Thomas, VP of strengthening solutions at Structural, more than1.85 million m2 of carbon fiber composites already have been installed in reinforced concrete buildings in the U.S. alone. Structure’s carbon fiber/epoxy V-Wrap material is one solution of several available solutions, made with a unidirectional fabric of intermediate-modulus carbon fibers, which becomes an integral part of a concrete member via bonding with epoxy resin. The ease and speed of application of such systems compared to using traditional materials often helps reduce overall project costs, and in some cases, even makes an otherwise unworkable project possible.
The badly corroded steel monopole had been condemned and revised building codes prevented construction of a replacement on the building. Composite repair provided the only practical solution. The repair featured unidirectional carbon fiber, aligned parallel to the tower axis and carefully bonded to the entire outer surface, followed by outer hoop wraps of uni carbon over 40% of its surface, to ensure structural integrity. A cement-based grout injection followed, to provide additional stiffness to the monopole. Once stabilized, the tower was wrapped with Comptek’s water-activated polyurethane prepreg as an outer weather-tight layer.
Composites also can be used to repair product pipelines, such as natural gas and petroleum pipes at oil refineries and offshore platforms. One example is Henkel’s (Düsseldorf, Germany) Loctite Composite Repair System for steel pipes and pipelines, which has been certified by DNV GL to the global quality standard ISO/TS 24817. Thus, the system is qualified for repairing oil and gas pipelines and pipework that carry petrochemicals.
Continuous exposure of a pipe’s internal and external surfaces to corrosive attack from weather, mechanical stress and chemicals makes it necessary for pipeline operators to regularly deal with cracks, holes, fractures and leaks. The Loctite repair system includes a corrosion inhibitor, surface filler and high-strength glass/carbon fiber tape that is pre-impregnated with temperature-resistant epoxy resin. The resin is reportedly engineered specifically for bonding to steel substrates. The repaired section is then sealed with a spray able ceramic topcoat. Corroded pipes including not only straight sections, but bends, tees, reducers and flanges can be repaired in situ, without any interruption in operations even in situations where pipes are subject to high internal operating pressures. Thus, operators can carry out repairs but avoid work stoppages and resulting commercial losses. Moreover, the quality of the repair is such that it can increase the useful life of steel pipelines by up to 20 years.
Composites also are used to rehabilitate underground storage tanks without the time and expense of excavation. A number of companies provide internal tank lining services, using various fiberglass products to meet the requirements for double-walled tanks and interstitial monitoring now in place.
Carbon fiber
Steel-reinforced concrete is among the most widely used building materials in the world, but it faces potential threats, including damage from seismic events, and cracking due to temperature-related expansion and contraction. The latter is common, and permits moisture invasion, and the resulting corrosion and expansion of steel rebar deteriorates the concrete, resulting eventually in loss of structural capacity. In many cases, distressed structures can be repaired. They also can be strengthened to comply with changes in load demands or code revisions. Further, design or construction deficiencies can be corrected to improve load-bearing capabilities.
Carbon fiber composites play a major role in such repairs and upgrades, and do so at significantly less cost than new construction. According to Jay Thomas, VP of strengthening solutions at Structural (Hanover, Md., a Structural Group company), more than 20 million ft2 (1.85 million m2) of carbon fiber already have been installed in reinforced concrete buildings in the U.S. alone. Structural, in the business for nearly 40 years, comes well-prepared. “We take a lot of factors into account, including constructability, aesthetics and cost, and essentially, reverse engineer the building’s structure to improve performance,” he asserts.
Percentage of ultimate capacity
Both structural repair and structural strengthening require knowledge of the global behaviour of the entire building,” Thomas explains, noting that the task is sometimes complicated by the fact that the engineer has no first-hand knowledge of how the structure was originally designed or built. “A design must be tailored for each specific situation and its load conditions.” To that end, Structural’s engineers first conduct a comprehensive inspection. The structure’s elements are catalogued as to type (e.g., round vs. rectangular columns, slab construction, etc.) and condition, and compared to the original construction drawings, if available. A key step is locating and mapping the reinforcing steel inside the concrete, which is accomplished with ground-penetrating radar (GPR) equipment. Other non-destructive testing methods (e.g., pin testing) are used to measure the concrete’s resistance to penetration. Typically, cores extracted from the concrete are subject to microscopic examination and compressive strength tests in the laboratory. The goal is to identify, quantify and evaluate compression strength problem areas.
The investigative team uses the site inspection data to build a virtual layout of the building, using modeling software developed for repair/strengthening applications. SAP2000 from Computers & Structures Inc. (Walnut Creek, Calif.) is one of several programs used. “What the modeling ultimately helps determine is, can the building withstand the load requirements desired by the customer? Are required upgrades constructable and cost-effective, when compared to replacement?” says Thomas. Answers to these questions help the team develop and cost a specific repair or rehab strategy.
“Carbon fiber is one of several solutions we can implement,” he points out. If the modelling shows that the required actual load demand exceeds the existing capacity by more than 40 percent, the strengthening options must be able to carry significant primary loads. Options include bonded steel plates and enlargement of existing concrete structural members. However, if the increase in load conditions is less than 40 percent, the most cost-effective solution, he contends, is Structure’s trademarked carbon fiber/epoxy V-Wrap material. Made with a proprietary unidirectional fabric of intermediate-modulus carbon fibers supplied by Toray Industries Inc. (Tokyo, Japan, and Decatur, Ala.), the material can be used alone or in combination with the options listed above. Labour costs typically drive strengthening projects, so the cost of the carbon isn’t a factor, Thomas claims, and the relative ease of application can actually help reduce project costs.
Composite action
V-Wrap works because it becomes an integral part of a concrete member via bonding, resulting in what Thomas calls “composite” action. The term is a reference to the fact that the carbon/epoxy laminate and the concrete column or slab to which it’s applied act together to bear applied loads. To achieve this effect, however, surface preparation is crucial, Thomas stresses. Grit-blasting and grinding with specialized abrasive disks (with the dust and debris vacuumed away) ensure a clean surface on undamaged concrete. “The concrete is like a big, hard sponge,” he explains. “You’ve got to open the pores and get the resin to fully penetrate to achieve an effective bond.”
Dry V-Wrap fabric sheets are wet-out at the job site, using Structure’s in-house-designed field saturators. (A saturator dips fabric in a resin bath and then passes it through precisely gapped rollers and/or scrapers, to yield a wet layup with a precise and project-specific resin content, without air bubbles that could result in voids.)
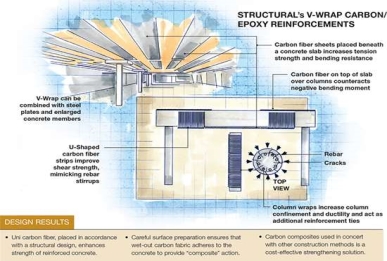
Where and how V-Wrap is applied is guided by Structure’s extensive experience, the modelling results and, in some cases, limitations imposed by fire codes. As shown in the drawing (top left), V-Wrap material placed beneath a horizontal slab increases its tension strength and bending resistance; strips are placed on the slab’s bottom surface with the fiber direction parallel to the slab’s steel rebar: “The V-Wrap is mimicking the action of the existing reinforcing steel, and augmenting its strength,” he explains.
Fiber sheet placed on a slab’s upper surface over supporting columns, for example counteract the slab’s negative bending moment. For additional beam shear capacity, typically where a beam is connected to a column, V-Wrap is applied to the beam in a “U” shape that mimics the beam’s steel shear stirrups. The stirrups and fiber prevent or minimize formation of 45° shear cracks that can occur if the beam/column intersection is overloaded. Finally, carbon fiber is exceptional at increasing confinement and ductility in concrete columns. When wrapped horizontally around the column, the fiber wrap acts as additional reinforcing ties around the column circumference.
Thomas cautions that the effects of strengthening local structural elements must be analysed carefully to determine their influence on the structure’s global behaviour. “Increasing the bending capacity of a structural element may overstress it in shear or, possibly, affect the surrounding elements, and lead to even bigger problems or even localized failure,” Thomas notes, pointing out that carbon fiber’s effectiveness depends on the original stiffness of the structural element to which it is applied. “Flexural strengthening is most effective on concrete members that were originally lightly or moderately reinforced,” he explains. Adding carbon fiber to an already very stiff element doesn’t contribute much, because the element must deflect to engage the bonded fiber. Structural follows guidance from the American Concrete Institute’s ACI 440.2R-08, Guide for the Design and Construction of Externally Bonded FRP Strengthening Systems for Strengthening Concrete Structures (2008), which, in essence, discourages the use of excessive FRP. “Full-scale testing has shown that more is not better,” he notes. “Too many plies create stresses that exceed the concrete’s tensile capacity and can actually cause the FRP to fail by peeling it away from the concrete under high loads.
To ensure that the upgraded structure will perform in service as predicted, load tests, both cyclic and monotonic (steadily increasing load), can be performed before and after the strengthening, using hydraulic actuators, to validate that the addition of the carbon fiber has achieved the required increase in capacity. And to verify that V-Wrap is bonded securely, Structural conducts pull-off adhesion tests.
References:
1. Patil, B.S. (1974), Legal Aspects of Building and Engineering Contract
2. The National Building Code, BIS, (2017)
3. RERA Act, (2017)
4. Meena Rao (2006), Fundamental concepts in Law of Contract, 3rd Edn. Professional Offset
5. Chandiramani, Neelima (2000), The Law of Contract: An Outline, 2nd Edn. Avinash Publications Mumbai
6. Avtarsingh (2002), Law of Contract, Eastern Book Co.
7. Dutt (1994), Indian Contract Act, Eastern Law House
8. Anson W.R. (1979), Law of Contract, Oxford University Press
9. Kwatra G.K. (2005), The Arbitration & Conciliation of Law in India with case law on UNCITRAL Model Law on Arbitration, Indian Council of Arbitration
10. Avtarsingh (2005), Law of Arbitration and Conciliation, Eastern Book Co.
11. Wadhera (2004), Intellectual Property Rights, Universal Law Publishing Co.
12. P. S. Narayan (2000), Intellectual Property Rights, Gogia Law Agency
13. T. Ramappa (2010), Intellectual Property Rights Law in India, Asia Law House
14. Bare text (2005), Right to Information Act
15. O.P. Malhotra, Law of Industrial Disputes, N.M. Tripathi Publishers
16. K.M. Desai(1946), The Industrial Employment (Standing Orders) Act
17. Rustamji R.F., Introduction to the Law of Industrial Disputes, Asia Publishing House 18. Vee, Charles & Skitmore, Martin (2003) Professional Ethics in the Construction Industry, Engineering Construction and Architectural Management, Vol.10, Iss. 2, pp 117-127, MCB UP Ltd
18. American Society of Civil Engineers (2011) ASCE Code of Ethics – Principles Study and Application
19. Ethics in Engineering- M.W.Martin& R.Schinzinger, McGraw-Hill
20. Engineering Ethics, National Institute for Engineering Ethics, USA
21. www.ieindia.org
22. Engineering ethics: concepts and cases – C. E. Harris, M.S. Pritchard, M.J.Rabins
23. Resisting Bureaucratic Corruption: Alacrity Housing Chennai (Teaching Case Study) -S. Ramakrishna Velamuri -CEIBS
24. CONSTRUCTION CONTRACTS, http://www.jnormanstark.com/contract.htm
25. Internet and Business Handbook, Chap 4, CONTRACTS LAW, http://www.laderapress.com/laderapress/contractslaw1.html