Unit-2
Petrology
Petrology is the branch of geology that studies rocks and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together because they both contain heavy use of chemistry, chemical methods, and phase diagrams. Sedimentary petrology is, on the other hand, commonly taught together with stratigraphy because it deals with the processes that form sedimentary rock.
Background
Lithology was once approximately synonymous with petrography, but in current usage, lithology focuses on macroscopic hand-sample or outcrop-scale description of rocks while petrography is the specialty that deals with microscopic details.
In the petroleum industry, lithology, or more specifically mud logging, is the graphic representation of geological formations being drilled through, and drawn on a log called a mud log. As the cuttings are circulated out of the borehole they are sampled, examined (typically under a 10× microscope), and tested chemically when needed.
Methodology
Petrology utilizes the fields of mineralogy, petrography, optical mineralogy, and chemical analysis to describe the composition and texture of rocks. Petrologists also include the principles of geochemistry and geophysics through the study of geochemical trends and cycles and the use of thermodynamic data and experiments to better understand the origins of rocks.
Branches
There are three branches of petrology, corresponding to the three types of rocks: igneous, metamorphic, and sedimentary, and another dealing with experimental techniques:
The conceptual framework for rock development is provided by a model rock cycle, which is introduced as consisting of primary and secondary loops, corresponding to conventional distinctions between igneous and sedimentary rocks.The formation of primary minerals from magma and the importance of silicates lead naturally into igneous processes and landforms. Alteration and resorption of oceanic crust represent the shortest route through the rock cycle, and there are also strong associations between magma emplacement, subduction, and metamorphism.
The rock cycle
The oceanic rock cycle
Specific gravity (G) is defined as the ratio between the weight of a substance and the weight of an equal volume of water at 4 °C (39 °F). Thus a mineral with a specific gravity of 2 weighs twice as much as the same volume of water. Since it is a ratio, specific gravity has no units.
The specific gravity of a mineral depends on the atomic weights of all its constituent elements and how the atoms (and ions) are packed together. In mineral series whose species have essentially identical structures, those composed of elements with higher atomic weight have higher specific gravities. If two minerals (as in the two polymorphs of carbon, namely graphite and diamond) have the same chemical composition, the difference in specific gravity reflects variation in internal packing of the atoms or ions (diamond, with a G of 3.51, has a more densely packed structure than graphite, with a G of 2.23).
Measurement of the specific gravity of a mineral specimen requires the use of a special apparatus. An estimate of the value, however, can be obtained by simply testing how heavy a specimen feels. Most people, from everyday experience, have developed a sense of relative weights for even such objects as nonmetallic and metallic minerals. For example, borax (G = 1.7) seems light for a nonmetallic mineral, whereas anglesite (G = 6.4) feels heavy. Average specific gravity reflects what a nonmetallic or metallic mineral of a given size should weigh. The average specific gravity for nonmetallic minerals falls between 2.65 and 2.75, which is seen in the range of values for quartz (G = 2.65), feldspar (G = 2.60 to 2.75), and calcite (G = 2.72). For metallic minerals, graphite (G = 2.23) feels light, while silver (G = 10.5) seems heavy. The average specific gravity for metallic minerals is approximately 5.0, the value for pyrite. With practice using specimens of known specific gravity, a person can develop the ability to distinguish between minerals that have comparatively small differences in specific gravity by merely lifting them.
Although an approximate assessment of specific gravity can be obtained by the hefting of a hand specimen of a specific monomineral, an accurate measurement can only be achieved by using a specific gravity balance. An example of such an instrument is the Jolly balance, which provides numerical values for a small mineral specimen (or fragment) in the air as well as in water. Such accurate measurements are highly diagnostic and can greatly aid in the identification of an unknown mineral sample.
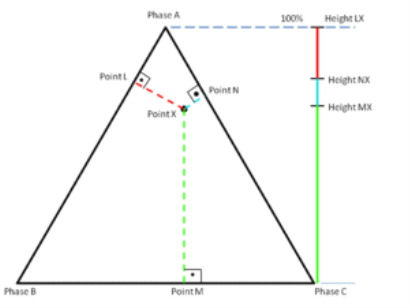
A ternary plot, ternary graph, triangle plot, simplex plot, Gibbs triangle, or de Finetti diagram is a barycentric plot on three variables that sum to a constant. It graphically depicts the ratios of the three variables as positions in an equilateral triangle. It is used in physical chemistry, petrology, mineralogy, metallurgy, and other physical sciences to show the compositions of systems composed of three species. In population genetics, it is often called a de Finetti diagram. In game theory, it is often called a simplex plot. Ternary plots are tools for analyzing compositional data in the three-dimensional case.
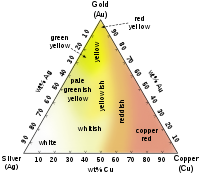
Fig. 2.1Approximate colors of Ag–Au–Cu alloys in jewelry making
In a ternary plot, the values of the three variables a, b, and c must sum to some constant, K. Usually, this constant is represented as 1.0 or 100%. Because a + b + c = K for all substances being graphed, any one variable is not independent of the others, so only two variables must be known to find a sample's point on the graph: for instance, c must be equal to K − a − b. Because the three numerical values cannot vary independently—there are only two degrees of freedom—it is possible to graph the combinations of all three variables in only two dimensions.
Reading values on the ternary plot
The advantage of using a ternary plot for depicting chemical compositions is that three variables can be conveniently plotted in a two-dimensional graph. Ternary plots can also be used to create phase diagrams by outlining the composition regions on the plot where different phases exist.
Every point on a ternary plot represents a different composition of the three components.
A parallel to a side of the triangle is the locus of points representing systems with constant chemical composition in the component situated in the vertex as opposed to the side.
There are three common methods used to determine the ratios of the three species in the composition.
The first method is an estimation based upon the phase diagram grid. The concentration of each species is 100% (pure phase) in each corner of the triangle and 0% at the line opposite it. The percentage of a specific species decreases linearly with increasing distance from this corner, as seen in figures 3–8. By drawing parallel lines at regular intervals between the zero line and the corner (as seen in the images), fine divisions can be established for easy estimation of the content of a species. For a given point, the fraction of each of the three materials in the composition can be determined by the first.
For phase diagrams that do not possess grid lines, the easiest way to determine the composition is to set the altitude of the triangle to 100% and determine the shortest distances from the point of interest to each of the three sides. By Viviani's theorem, the distances (the ratios of the distances to the total height of 100%) give the content of each of the species, as shown in figure 2.2.
The third method is based upon a larger number of measurements but does not require the drawing of perpendicular lines. Straight lines are drawn from each corner, through the point of interest, to the opposite side of the triangle. The lengths of these lines, as well as the lengths of the segments between the point and the corresponding sides, are measured individually. Ratios can then be determined by dividing these segments by the entire corresponding line as shown in figure 2.3 (The sum of the ratios should add to 1).
Figure 2.2. Altitude method
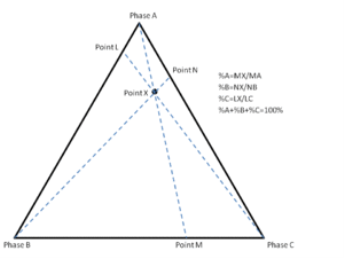
Figure 2.3 Intersection method
Figure 2.4 An example ternary diagram, without any points, plotted.
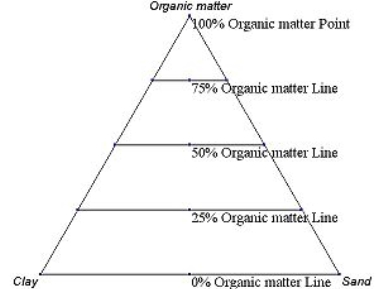
Figure 2.5 An example ternary diagram, showing increments along the first axis.
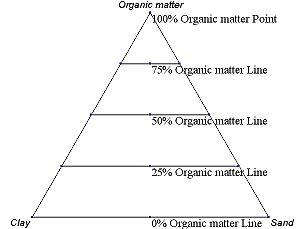
Figure 2.6 An example ternary diagram, showing increments along the second axis.
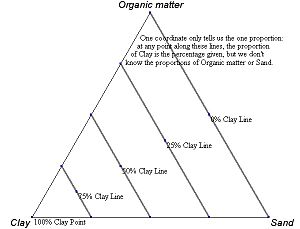
Figure 2.7 An example ternary diagram, showing increments along the third axis.
Figure 2.8 Empty ternary plot
Igneous rock is formed when liquid rock freezes into solid rock. This molten material is called magma when it is in the ground and lava when it is on the surface. Only the Earth’s outer core is liquid; the Earth’s mantle and crust are naturally solid. However, there are a few minor pockets of magma that form near the surface where geologic processes cause melting. It is this magma that becomes the source for volcanoes and igneous rocks.

Fig. 2.10 The lava flow in Hawaii
Lava cools quickly on the surface of the earth and forms tiny microscopic crystals. These are known as fine-grained extrusive, or volcanic, igneous rocks. Extrusive rocks are often vesicular, filled with holes from escaping gas bubbles. Volcanism is the process in which lava erupts. Depending on the properties of the lava that is erupted, the volcanism can be drastically different, from smooth and gentle to dangerous and explosive. This leads to different types of volcanoes and different volcanic hazards.
In contrast, magma that cools slowly below the earth’s surface forms larger crystals which can be seen with the naked eye. These are known as coarse-grained intrusive, or plutonic, igneous rocks. This relationship between cooling rates and grain sizes of the solidified minerals in igneous rocks is important for interpreting the rock’s geologic history.
Volcanic eruptions produce three types of materials: gas, lava, and fragmented debris called tephra.
Volcanic Gas
Magma contains gas. At high pressures, the gases are dissolved within magma. However, if the pressure decreases, the gas comes out of the solution, forming bubbles. This process is analogous to what happens when a pop bottle is opened. Pop is bottled under pressure, forcing carbon dioxide gas to dissolve into the fluid. As a result, a bottle of pop that you find on the supermarket shelf will have few to no bubbles. If you open the bottle, you decrease the pressure within it. The pop will begin to fizz as carbon dioxide gas comes out of the solution and forms bubbles.
The main component of volcanic gas emissions is water vapor, followed by carbon dioxide (CO2), sulfur dioxide (SO2), and hydrogen sulfide (H2S).
Volcanoes release gases when erupt, and through openings called fumaroles (Figure 11.7). They can also release gas into soil and groundwater.
 Figure 2.11 A fumarole at PuʻuʻŌʻō Crater, Hawaii.
Figure 2.11 A fumarole at PuʻuʻŌʻō Crater, Hawaii.
Lava
The ease with which lava flows and the structures it forms depends on how much silica and gas the lava contains. The more silica, the more polymerization (formation of long molecules) occurs, stiffening the lava. The stiffness of lava is described in terms of viscosity– lava that flows easily has low viscosity, and lava that is sticky and stiff has high viscosity.
In general, high-silica lava contains more gas than low-silica lava. When the gas forms into bubbles, viscosity increases further. Consider the pop analogy again. If you were to shake the bottle vigorously and then open it, the pop would come gushing out in a thick, frothy flow. In contrast, if you took care to not shake the bottle before opening it, you could pour out a thin stream of fluid.
Chemical Composition Affects the Thickness and Shape of Lava Flows
The thickness and shape of a lava flow depend on its viscosity. The greater the viscosity, the thicker the flow, and the shorter the distance it travels before solidifying. Highly viscous lava might not flow very far at all and simply accumulate as a bulge, called a lava dome, in a volcano’s crater. Figure 2.12 shows a dome formed from rhyolitic lava in the crater of Mt. St. Helens.

Figure 2.12 Lava dome in the crater of Mt. St. Helens.
Less viscous rhyolitic lava can travel further, as with the thick flow in Figure 2.13 (right). The left of Figure 2.13 shows thin streams of freely-flowing, low-silica, low-viscosity basaltic lava.
 Figure 2.13 Lava flows.
Figure 2.13 Lava flows.
Low-viscosity basaltic lava flows may travel extended distances if they move through conduits called lava tubes. These are tunnels within older solidified lava flows. Figure 2.14 (top) shows a view into a lava tube through a hole in the overlying rock, called a skylight. Figure 2.14 (bottom) shows the interior of a lava tube, with a person for scale. Lava tubes form naturally and readily because flowing mafic lava preferentially cools near its margins, forming solid lava levées that eventually close over the top of the flow. Lava within tubes can flow for 10s of km because the tubes insulate the lava from the atmosphere and slow the rate at which the lava cools. The Hawai’ian volcanoes are riddled with thousands of old, drained lava tubes, some as long as 50 km.
 Figure 2.14 Lava tubes.
Figure 2.14 Lava tubes.
Pyroclastic Materials
The pop bottle analogy illustrates another key point about gas bubbles in the fluid, which is that the bubbles can propel fluid. In the same way that shaking a pop bottle to make more bubbles will cause pop to gush out when the bottle is opened, gas bubbles can violently propel lava and other materials from a volcano, creating an explosive eruption.
Collectively, loose material thrown from a volcano is referred to as tephra. Individual fragments are referred to in general terms as pyroclasts, so sometimes tephra is also referred to as pyroclastic debris. Pyroclasts are classified according to size.
Volcanic Ash
Particles less than 2 mm in diameter are called volcanic ash. Volcanic ash consists of small mineral grains and glass. Figure 2.15 shows volcanic ash on three scales: in the upper left is ash from the 2010 eruption of Eyjafjallajökull in Iceland. The image was taken with a scanning electron microscope at approximately 1000 times magnification. In the upper right is ash from the 1980 eruption of Mt. St. Helens, collected in Yakima, Washington, about 137 km northeast of Mt. St. Helens. Individual particles are under 1 mm in size. Figure 2.15 (bottom) shows a village near Mt. Merapi in Indonesia dusted in ash after an eruption in 2010.
Lapilli
Fragments with dimensions between 2 mm and 64 mm are classified as lapilli. Figure 2.16 (upper left) shows lapilli at the ancient city of Pompeii, which was buried when Mt. Vesuvius erupted in 79 C.E. Figure 2.16 (lower left) is a form of lapilli called Pele’s tears, named after the Hawai’ian deity Pele. Pele’s tears form when droplets of lava cool quickly as they are flung through the air. Rapidly moving through the air may draw the Pele’s tears out into long threads called Pele’s hair (Figure 2.16, right). The dark masses in Figure 2.16 (right) within Pele’s hair are Pele’s tears.
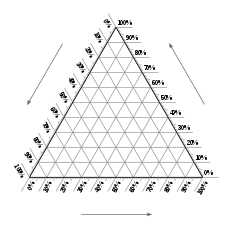 Figure 2.16 Lapilli are pyroclasts ranging between 2 mm and 64 mm in size
Figure 2.16 Lapilli are pyroclasts ranging between 2 mm and 64 mm in size
Blocks and Bombs
Fragments larger than 64 mm are classified as blocks or bombs, depending on their origin. Blocks are solid fragments of the volcano that form when an explosive eruption shatters the pre-existing rocks.
Bombs form when lava is thrown from the volcano and cools as it travels through the air. Traveling through the air may cause the lava to take on a streamlined shape, as with the example in Figure 2.17
 Figure 2.17 Volcanic bomb with a streamlined shape.
Figure 2.17 Volcanic bomb with a streamlined shape.
There are four types of eruptions with properties determined mostly by the silica content of the magma, and the amount of gas it contains. In order of increasing explosiveness,these are Hawai’ian, Strombolian, Vulcanian, and Plinian eruptions. Any composition of magma can have an explosive eruption if the magma suddenly encounters water. Hot magma contacting groundwater or seawater causes the water to flash to steam. Explosive eruptions driven by water are called hydrovolcanic (or phreatic) eruptions.
Hawai‘ian Eruptions
Hawai‘ian eruptions are named after the characteristic eruptions of volcanoes of the Hawai‘ian islands. Hawai‘ian eruptions are effusive (flowing) rather than explosive because they erupt low-viscosity basaltic lava. Hawai‘ian eruptions form shield volcanoes and can also take the form of fissure eruptions. Fissure eruptions occur when lava erupts from long cracks in the ground rather than from a central vent.
Figure 2.18 shows examples from two eruptions on of Hawai‘i. In the upper left and right are images from the November 1959 eruption of KīlaueaIki Crater. The upper left shows a fissure eruption and effusive flow of lava. Burning trees appear as bright spots toward the bottom of the photo. Figure 2.18 (right) shows a lava fountain reaching 425 m above KīlaueaIki Crater. U. S. Geological Survey scientists reported that volcanic bombs up to 60 cm across smashed the guard rail and dented the asphalt on the road. Figure 2.18 (lower left) shows Hawaiian Volcano Observatory (HVO) scientists making a quick getaway, with lava fountains from Mauna Loa Volcano in the background.
The photographs of the KīlaueaIki Crater and Mauna Loa Volcano eruptions make the point that while Hawai‘ian eruptions are considered “gentle” eruptions, this is a relative term. “Gentle” eruptions range from lava flows that can be safely sampled by trained personnelto lava fountains that soar hundreds of meters above the treetops and rain large and dangerous rocks upon the surroundings.
Strombolian Eruptions
Strombolian eruptions, named for Mt. Stromboli in Italy, occur when basaltic lava has a higher viscosity and higher gas content. The sticky lava is ejected in loud, violent, but short-lived splattery eruptions. Clumps of gas-rich lava thrown 10s to 100s of meters in the air accumulate as scoria in a pile around the vent, forming cinder cones. Figure 2.19 shows a strombolian eruption in the crater of Mt. Etna. A smaller cinder cone is forming around the vent as lava sputters out of it.
 Figure 2.19 Strombolian eruption of Mt. Etna.
Figure 2.19 Strombolian eruption of Mt. Etna.
Vulcanian Eruptions
Vulcanian eruptions get their name from the volcanic Italian island of Vulcano, which itself takes the name of the Roman god of fire, Vulcan. In Roman mythology, Vulcan was the maker of armor and weaponry for the gods, and volcanic eruptions were attributed to him working in his forge.
Vulcanian eruptions are far more explosive than Strombolian eruptions and can blast tephra and gas to a height of 5 to 10 km. The explosiveness is related to a build-up of pressure as the higher viscosity of intermediate silica content lava restricts the escape of gas. Vulcanian eruptions produce large quantities of ash in addition to blocks and bombs.
The Vulcanian eruption of Mt. Pelée on the island of Martinique in 1902 resulted in the first detailed documentation by geologists of a devastating phenomenon that is now referred to as a pyroclastic flow (Fig. 2.20). Volcanic debris from the collapse of a lava dome on Mt. Pelée combined with hot gas to form a searing avalanche that raced down the mountain, over the city of St. Pierre, and into the harbor.
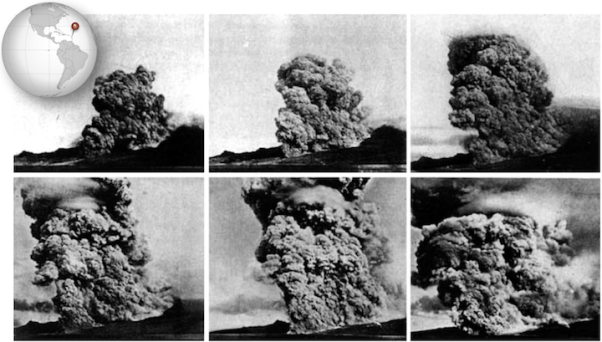 Figure 2.20 A series of photos taken by Alfred Lacroix during the eruption of Mt. Pelée
Figure 2.20 A series of photos taken by Alfred Lacroix during the eruption of Mt. Pelée
The French geologist Alfred Lacroix described what he saw as a “nuée ardente,” or thick fiery cloud.
In some cases, pyroclastic flows travel at speeds greater than 700 km/h. They can travel rapidly because they behave like fluid, and can also ride on a cushion of hot gas. The ruins of St. Pierre look as though the top of the city were shaved off, and that is effectively what happened as the pyroclastic flow rushed across it, buoyed by gas.
The vast majority of fatalities from the eruption were caused by the heat of pyroclastic flow. Examination of the ruins of St. Pierre revealed that glass had melted, but copper had not, putting the temperature at between 700 ºC and 1000 ºC (1292 ºF to 1832 ºF).
Plinian Eruptions
Plinian eruptions are explosive eruptions of intermediate to felsic lava and can form eruptive columns up to 45 km high. The origin of the name is the eruption of Vesuvius in 79 CE, which buried the towns of Pompeii and Herculaneum. The Roman admiral Gaius Plinius Secundus, also known as Pliny the Elder, attempted a rescue mission when he saw the column of ash and debris above Vesuvius, but died of unknown causes without being able to reach Herculaneum.
A more recent Plinian eruption was that of Mt. Redoubt on April 21, 1990, shown in Figure 2.21. Pyroclastic flows resulted, as did lahars, landslides that formed when glaciers melted and turned volcanic ash into the mud. The shape of the eruptive column, with parts of the column appearing to spread out in flat layers at different levels, reflects differences in atmospheric characteristics.
 Figure 2.21 Plinian eruption of Mt. Redoubt in Alaska on April 21, 1990
Figure 2.21 Plinian eruption of Mt. Redoubt in Alaska on April 21, 1990
Hydrovolcanic (Phreatic) Eruptions
Hydrovolcanic eruptions can be far more explosive than Plinian eruptions. They occur when water in the form of groundwater, seawater, or even melting glacial ice or snow comes into contact with magma. The heat from the magma changes water suddenly to steam, which can expand to more than a thousand times the original volume of water. The sudden expansion results in an explosive force that can blast a volcano to pieces and create large amounts of volcanic ash.
In April of 2010, activity by the Icelandic volcano Eyjafjallajökull (Figure 2.22) melted the glacier above it, releasing large quantities of water and triggering a hydrovolcanic eruption. Ash rose in a plume 10 km high and was blown westward and into the skies over Europe. Volcanic ash can damage or destroy aircraft engines, so precaution was taken to prohibit air travel for 5 days. The enormous economic impact of stopping flights has led to numerous studies about the best way to deal with similar events with volcanic ash in the future.
 Figure 2.22 Hydrovolcanic eruption of Eyjafjallajökull in April of 2010.
Figure 2.22 Hydrovolcanic eruption of Eyjafjallajökull in April of 2010.
Hot springs and geysers are manifestations of volcanic activity. They result from the interaction of groundwater with magma or with solidified but still-hot igneous rocks at shallow depths.
Hot SpringsWhen hot water gently rises to the surface, it creates a hot spring. A hot spring forms where a crack in the Earth allows water to reach the surface after being heated underground. Many hot springs are used by people and animals as natural hot tubs. Some people believe that hot springs can cure illnesses. Hot springs are found all over the world, even in Antarctica.
GeysersGeysers are also created by water that is heated beneath the Earth’s surface. The water may become superheated by magma. It becomes trapped in a narrow passageway. The heat and pressure build as more water is added. When the pressure is too much, the superheated water bursts out onto the surface. This is a geyser.
Yellowstone National Park in the United States is one of the most famous areas of hot springs and geysers in the world. The total heat flux from these thermal features is estimated to be 300 megawatts (300 million watts). The last great eruption at Yellowstone occurred about 630,000 years ago when some 1,000 cubic km (240 cubic miles) of rhyolitic pumice and ash were ejected in huge pyroclastic flows and resulted in the formation of a caldera—a large circular or oval depression caused by the collapse of the surface following magma removal—approximately 45 by 75 km (28 by 47 miles) in size. Yellowstone Lake now occupies part of this giant caldera. Since that last great outburst, about 1,200 cubic km (288 cubic miles) of rhyolite lava flows and domes have erupted in numerous smaller events. The cooling roots of such past eruptions, or possibly the new intrusions of magma at shallow depth, are the heat sources for the Yellowstone hot springs and geysers.
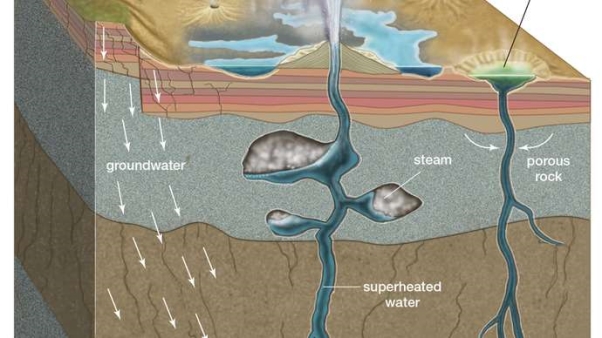
Fig 2.23
Groundwater percolates through the porous rock into fractures deep underground, where heat from a nearby magma chamber superheats the pressurized water to a temperature above the boiling point of water at surface pressure. In hot springs the rising superheated water is cooled below the boiling point by groundwater before reaching the surface. In geysers, the superheated water collects in underground pockets. There a small drop in pressure caused by the release of water at the surface flashes the superheated water into steam, which expands and ejects a column of steam and water into the air. When the supply of steam and hot water is exhausted, the spouting stops and the cycle begins again.
Types of Magma
Types of magma are determined by the chemical composition of the magma. Three general types are recognized:
Gases in Magmas
At depth in the Earth, nearly all magmas contain gas dissolved in the liquid, but the gas forms a separate vapor phase when pressure is decreased as magma rises toward the surface. This is similar to carbonated beverages which are bottled at high pressure. The high pressure keeps the gas in the solution in the liquid, but when pressure is decreased, like when you open the can or bottle, the gas comes out of the solution and forms a separate gas phase that you see as bubbles. Gas gives magmas their explosive character because the volume of gas expands as pressure is reduced. The composition of the gases in magma are:
The amount of gas in magma is also related to the chemical composition of the magma. Rhyolitic magmas usually have higher dissolved gas contents than basaltic magmas.
Temperature of Magmas
The temperature of magmas is difficult to measure (due to the danger involved), but laboratory measurement and limited field observation indicate that the eruption temperature of various magmas is as follows:
Viscosity of Magmas
Viscosity is the resistance to flow (opposite of fluidity). Viscosity depends primarily on the composition of the magma, and temperature.
Higher SiO2 (silica) content magmas have a higher viscosity than lower SiO2 content magmas (viscosity increases with increasing SiO2 concentration in the magma).
Lower temperature magmas have a higher viscosity than higher temperature magmas (viscosity decreases with increasing temperature of the magma).
Thus, basaltic magmas tend to be fairly fluid (low viscosity), but their viscosity is still 10,000 to 100,0000 times more viscous than water. Rhyolitic magmas tend to have an even higher viscosity, ranging between 1 million and 100 million times more viscous than water. (Note that solids, even though they appear solid have a viscosity, but it is very high, measured as the trillions of time the viscosity of water). Viscosity is an important property in determining the eruptive behavior of magmas.
Summary Table | |||||
Magma Type | Solidified Rock | Chemical Composition | Temperature | Viscosity | Gas Content |
Basaltic | Basalt | 45-55 SiO2 %, high in Fe, Mg, Ca, low in K, Na | 1000 – 1200 oC | 10 – 103 PaS | Low |
Andesitic | Andesite | 55-65 SiO2 %, intermediate in Fe, Mg, Ca, Na, K | 800 – 1000 oC | 103 – 105 PaS | Intermediate |
Rhyolitic | Rhyolite | 65-75 SiO2 %, low in Fe, Mg, Ca, high in K, Na. | 650 – 800 oC | 105 – 109 PaS | High |
Table 2.1
Based onthe depth of formation
Based on Chemical composition
Composition refers to a rock’s chemical and mineral make-up. For igneous rock, the composition is divided into four groups: felsic, intermediate, mafic, and ultramafic. These groups refer to differing amounts of silica, iron, and magnesium found in the minerals that make up the rocks. It is important to realize these groups do not have sharp boundaries in nature, but rather lie on a continuous spectrum with many transitional compositions and names that refer to specific quantities of minerals. As an example, granite is a commonly-used term but has a very specific definition that includes exact quantities of minerals like feldspar and quartz. Rocks labeled as ‘granite’ in laymen applications can be several other rocks, including syenite, tonalite, and monzonite.
Texture
When magma cools slowly large crystals form and rock forms phaneritic texture on the other hand if magma cools fast then small crystals form sometimes a glassy texture where no minerals form can be achieved this way. It is based on the textural difference that igneous rocks can be divided into either extrusive or intrusive rocks. Examples of both extrusive and extrusive rocks are given in Figure 2.24. Intrusive are rocks that form by magma solidifying before reaching the surface hence forming coarse-grained texture while extrusive are those that magma solidifies on the surface forming fine-grained rocks.
Colour
A rock with majorly dark minerals form mafic rocks but with more fractionation, during magma cooling, lighter-colored minerals can form based on the Bowens series. Based on this color difference the rocks can be either mafic or felsic in Figure 2.24 shows that as you move from right to left you have more ultra-mafic due to fractionation.
Composition
Igneous rocks can also be classified based on chemistry. This is mainly based on silica content as highlighted in Figure 2.24. When silica is above 75% main minerals that form are feldspars while with reduction of silica more mafic minerals form, hence the basis for rock difference.
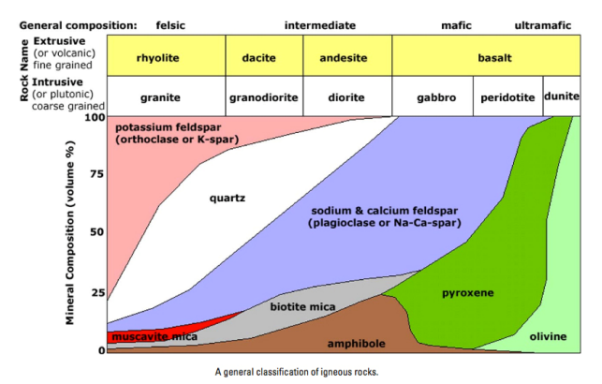
Figure 2.24: Types of igneous rocks based on texture, color, and chemistry
Texture
Phaneritic Texture
Phaneritic textured rocks are comprised of large crystals that are visible with or without a hand lens or binocular microscope. The entire rock is made up of large crystals, which are generally 1/2 mm to several centimeters in size; no fine matrix material is present. This texture forms by the slow cooling of magma deep underground in the plutonic environment.
Aphanitic Texture
Aphanitic texture consists of small crystals that cannot be seen by the eye with or hand lens. The entire rock is made up of small crystals, which are generally less than 1/2 mm in size. This texture results from rapid cooling in volcanic or hypabyssal (shallow subsurface) environments.
Porphyritic Texture
Porphyritic rocks are composed of at least two minerals having a conspicuous (large) difference in grain size. The larger grains are termed phenocrysts and the finer grains either matrix or groundmass (see the drawing below and image to the left). Porphyritic rocks are thought to have undergone two stages of cooling; one at the depth where the larger phenocrysts formed and a second at or near the surface where the matrix grains crystallized.
Glassy Texture
Glass-textured igneous rocks are non-crystalline meaning the rock contains no mineral grains. Glass results from cooling that is so fast that minerals do not have a chance to crystallize. This may happen when magma or lava comes into quick contact with much cooler materials near the Earth's surface. Pure volcanic glass is known as obsidian.
Vesicular Texture
This term refers to vesicles (cavities) within the igneous rock. Vesicles are the result of gas expansion (bubbles), which often occurs during volcanic eruptions. Pumice and scoria are common types of vesicular rocks.
Fragmental (Pyroclastic) Texture
Pyroclastic is rocks blown out into the atmosphere during violent volcanic eruptions. These rocks are collectively termed fragmental. If you examine a fragmental volcanic rock closely you can see why. You will note that it is comprised of numerous grains or fragments that have been welded together by the heat of volcanic eruption. If you run your fingers over the rock it will often feel grainy like sandpaper or sedimentary rock. You might also spot shards of glass embedded in the rock.
Mineralogical
The most abundant minerals are used as a prefix to a textural term. Thus, a schist containing biotite, garnet, quartz, and feldspar, would be called biotite-garnet schist. A gneiss containing hornblende, pyroxene, quartz, and feldspar would be called a hornblende-pyroxene gneiss. Schist containing porphyroblasts of K-feldspar would be called K-spar porphyroblastic schist.
Chemical
If the general chemical composition can be determined from the mineral assemblage, then a chemical name can be employed. For example, a schist with a lot of quartz and feldspar and some garnet and muscovite would be called garnet-muscovite quartzo-feldspathic schist. Schist consisting mostly of talc would be called talc-magnesian schist.
Texture
Most metamorphic textures involve foliation. Foliation is generally caused by a preferred orientation of sheet silicates. If a rock has a slatey cleavage as its foliation, it is termed a slate, if it has a phyletic foliation, it is termed a phyllite, if it has a shistose foliation, and it is termed schist. A rock that shows a banded texture without a distinct foliation is termed a gneiss. All of these could be porphyroblastic (i.e. could contain porphyroblasts). A rock that shows no foliation is called a hornfels if the grain size is small, and a granulite if the grain size is large and individual minerals can be easily distinguished with a hand lens.
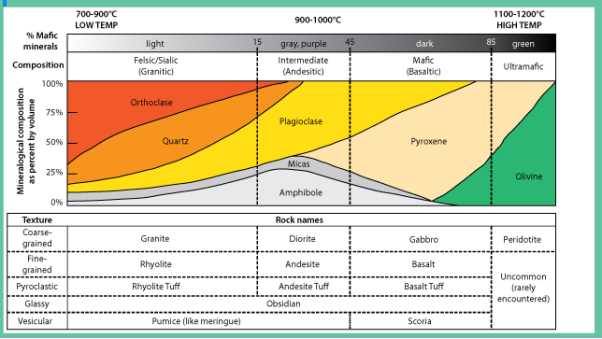
Fig 2.25
Igneous rocks can be divided into four categories based on their chemical composition: felsic, intermediate, mafic, and ultramafic. The diagram of Bowen’s reaction series shows that differences in chemical composition correspond to differences in the types of minerals within an igneous rock. Igneous rocks are given names based on the proportion of different minerals they contain. Figure 2.26 is a diagram with the minerals from Bowen’s reaction series and is used to decide which name to give an igneous rock.
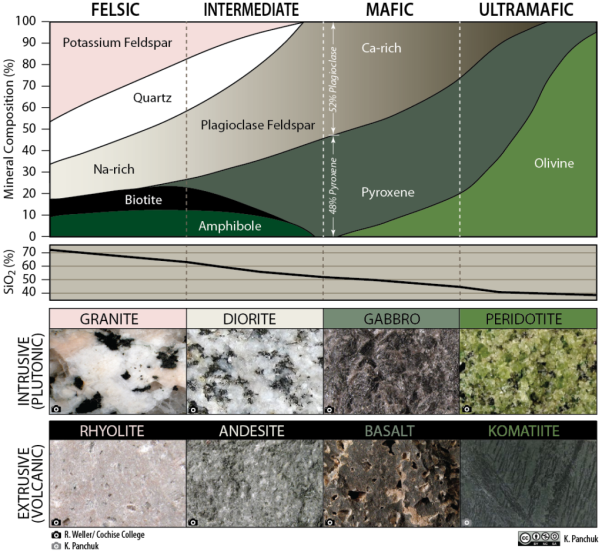 Figure 2.26 Classification diagram for igneous rocks.
Figure 2.26 Classification diagram for igneous rocks.
To see how Figure 2.26 works, first notice the scale in percent along the vertical axis. The interval between each tick mark represents 10% of the minerals within rock by volume. Igneous rock can be represented as a vertical line drawn through the diagram, and the vertical scale used to break down the proportion of each mineral it contains. For example, the arrows in the mafic field of the diagram represent a rock containing 48% pyroxene and 52% plagioclase feldspar. An igneous rock at the boundary between the mafic and ultramafic fields (marked with a vertical dashed line) would have approximately 20% olivine, 50% pyroxene, and 30% Ca-rich plagioclase feldspar by volume
IUGS Classification
In the field, a simple field-based classification must be used. This is usually based on mineralogical content and texture. For plutonic rocks, the IUGS system of classification can be used. For volcanic rocks, the following table can be used.
Simple Field Classification of Volcanic Rocks | ||
Rock Name | Essential Minerals* | Other Minerals (may or may not be present) |
Basalt | Olivine | Cpx, Opx, Plag. |
Basanite | Olivine + Feldspathoid (Nepheline/ Leucite) | Cpx, Plag. |
Andesite | No olivine, abundant Plagioclase | Cpx, Opx, Hornblende |
Trachyte | Sanidine + Plagioclase | Na-Cpx, Hornblende, Biotite |
Dacite | Plagioclase + Hornblende | Cpx, Opx, Biotite |
Rhyolite | Quartz | Sanidine, Biotite, Plag., Hornblende, Cpx, Opx |
* The amount of glass in the groundmass increases, in general, from the top to the bottom of the chart. | ||
Table 2.2
Once the rocks are brought back to the laboratory and thin sections can be made, these are examined, mineralogical content can be more precisely determined, and refinements in the mineralogical and textural classification can be made.
Chemical analyses can be obtained, and chemical classification, such as the LeBas et al., IUGS chemical classification of volcanic rocks (based on total alkalies [Na2O + K2O] vs. SiO2 diagram shown in fig 2.27)

Fig 2.27
Note that at each stage of the process, the classification may change, but it is important to keep in mind that each stage has limitations, and that classification at each stage is to describe the rock, not only for the individual investigator but anyone else. Thus, the classification scheme should be employed consistently so that later investigators can understand what you are talking about at each stage of the process.
Granite is the most widespread of igneous rocks, underlying much of the continental crust. Granite is intrusive igneous rock. Intrusive rocks form from molten material (magma) that flows and solidifies underground, where magma cools slowly. Eventually, the overlying rocks are removed, exposing the granite. Granites usually have a coarse texture (individual minerals are visible without magnification), because the magma cools slowly underground, allowing larger crystal growth.
Granites are most easily characterized as light-colored and coarse-grained as a result of cooling slowly below the surface. Color variation is a response to the percent of each mineral found in the sample. The crystals in granite provide a variety of mixed colors — feldspar (pink or red), mica (dark brown or black), quartz (clear pink, white, or black), and amphibole (black).
Granite is high in quartz (about 25%), feldspar, and mica. It is widely used for architectural facades, construction materials, ornamental stone, and monuments. Over 40% of dimension stone quarried is granite. Crushed granite is used as a durable construction material in asphalt and concrete used in highway and infrastructure projects.


Description
Granite is the most widespread of igneous rocks, underlying much of the continental crust. Granite is intrusive igneous rock. Intrusive rocks form from molten material (magma) that flows and solidifies underground, where magma cools slowly. Eventually, the overlying rocks are removed, exposing the granite. Granites usually have a coarse texture (individual minerals are visible without magnification), because the magma cools slowly underground, allowing larger crystal growth.
Granites are most easily characterized as light-colored and coarse-grained as a result of cooling slowly below the surface. Color variation is a response to the percent of each mineral found in the sample. The crystals in granite provide a variety of mixed colors — feldspar (pink or red), mica (dark brown or black), quartz (clear pink, white, or black), and amphibole (black).
Granite is high in quartz (about 25%), feldspar, and mica. It is widely used for architectural facades, construction materials, ornamental stone, and monuments. Over 40% of dimension stone quarried is granite. Crushed granite is used as a durable construction material in asphalt and concrete used in highway and infrastructure projects.
Relation to Mining
Granite is mined as either crushed stone or dimension stone mainly using open-pit mining methods. Crushed granite represents 16% of the total crushed stone produced in the U.S., and it is the second-most utilized crushed stone in the U.S. Crushed limestone is by far the most commonly used crushed rock in the U.S., representing 70% of total crushed rock consumption. Crushed granite is used in road construction and railroad beds. Larger pieces of granite are used to stabilize the land around roadways to minimize and even eliminate soil erosion.
Uses
There is an enormous abundance of granite throughout the United States, so it is not a surprise that a significant amount of granite is used in crushed stone applications. Crushed granite represents 16% of the total crushed stone produced in the U.S., and it is the second-most utilized crushed stone in the U.S. Crushed limestone is by far the most commonly used crushed rock in the U.S., representing 70% of total crushed rock consumption. The 16% represented by crushed granite (265,000 tons per year) is used in road construction and railroad beds. Larger pieces of granite are used to stabilize the land around roadways to minimize and even eliminate soil erosion.
Granite is used extensively as dimension stone. It is used in the construction of buildings, both as building blocks and as veneers on frame structures. Because it can be smoothed to a very high polish, granite has found extensive use in memorials, headstones, monuments, carved decorations on buildings, statues, and the like. Approximately 1.5 million tons of dimension stone are produced annually in the United States. Of this, granite accounts for over 400,000 tons (27%), second only to limestone.
Rhyolite is an extrusive igneous rock with very high silica content. It is usually pink or gray with grains so small that they are difficult to observe without a hand lens. Rhyolite is made up of quartz, plagioclase, and sanidine, with minor amounts of hornblende and biotite. Trapped gases often produce vugs in the rock. These often contain crystals, opal, or glassy material.
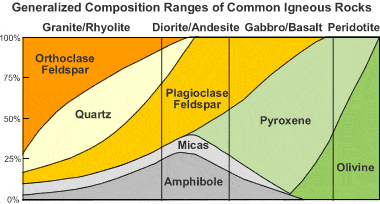
Fig 2.28
Igneous rock composition chart: This chart shows that rhyolite is typically composed of orthoclase, quartz, plagioclase, micas, and amphiboles.
Many rhyolites form from granitic magma that has partially cooled in the subsurface. When these magmas erupt, a rock with two-grain sizes can form. The large crystals that formed beneath the surface are called phenocrysts, and the small crystals formed at the surface are called groundmass.
Rhyolite usually forms in continental or continent-margin volcanic eruptions where granitic magma reaches the surface. Rhyolite is rarely produced at oceanic eruptions.
Fig 2.29
Rhyolite Porphyry: Several specimens of rhyolite porphyry, each about three inches across.
Eruptions of Granitic Magma
Eruptions of granitic magma can produce rhyolite, pumice, obsidian, or tuff. These rocks have similar compositions but different cooling conditions. Explosive eruptions produce tuff or pumice. Effusive eruptions produce rhyolite or obsidian if the lava cools rapidly. These different rock types can all be found in the products of a single eruption.
Eruptions of granitic magma are rare. Since 1900 only three are known to have occurred. These were at St. Andrew Strait Volcano in Papua New Guinea, Novarupta Volcano in Alaska, and Chaiten Volcano in Chile.
Granitic magmas are rich in silica and often contain up to several percent gases by weight. As these magmas cool, the silica starts to connect into complex molecules. This gives the magma a high viscosity and causes it to move very sluggishly.
The high gas content and high viscosity of these magmas are perfect for producing an explosive eruption. The viscosity can be so high that the gas can only escape by blasting the magma from the vent.
Granitic magmas have produced some of the most explosive volcanic eruptions in Earth's history. Examples include Yellowstone in Wyoming, Long Valley in California, and Valles in New Mexico. The sites of their eruption are often marked by large calderas.
Lava Domes
Sluggish rhyolitic lava can slowly exude from a volcano and pile up around the vent. This can produce a mound-shaped structure known as a "lava dome." Some lava domes have grown to a height of several hundred meters.
Lava domes can be dangerous. As additional magma extrudes, the brittle dome can become highly fractured and unstable. The ground can also change slope as the volcano inflates and contracts. This activity can trigger a dome collapse. A dome collapse can lower the pressure on the extruding magma. This sudden lowering of pressure can result in an explosion. It can also result in a debris avalanche of material falling from the tall collapsing dome. Many pyroclastic flows and volcanic debris avalanches have been triggered by a lava dome collapse.

Fig 2.30
Fire Opal is sometimes found filling cavities in rhyolite. This specimen of rhyolite has multiple vugs filled with gemmy transparent orange fire opal. This material can be cut into beautiful cabochons and is sometimes faceted when it is transparent or even translucent. Famous deposits of this type of fire-opal-in-rhyolite are found in Mexico. This photo is used here through a Creative Commons license. It was produced by Didier Descouens.
Rhyolite and Gemstones
Many gem deposits are hosted in rhyolite. These occur for a logical reason. The thick granitic lava that forms rhyolite often cools quickly while pockets of gas are still trapped inside of the lava. As the lava quickly cools, the trapped gas is unable to escape and forms cavities known as "vugs." Later, when the lava flow has cooled and hydrothermal gases or groundwater move through, the material can precipitate in the vugs. This is how some of the world's best deposits of red beryl, topaz, agate, jasper, and opal are formed. Gem hunters have learned this and are always on the lookout for vuggy rhyolite.
Rhyolite Arrowheads: Rhyolite was often used to make stone tools and weapons when more suitable materials were not available. It has been fashioned into scrapers, hoes, axe heads, spear points, and arrowheads.
Uses of Rhyolite
Rhyolite is a rock that is rarely used in construction or manufacturing. It is often vuggy or highly fractured. Its composition is variable. When better materials are not locally available, rhyolite is sometimes used to produce crushed stone. People have also used rhyolite to manufacture stone tools, particularly scrapers, blades, and projectile points. It was probably not their material of choice, but a material used out of necessity.
Felsite is a term that has long been generally used by geologists, especially in England, to designate fine-grained igneous rocks of acid (or subacid) composition. As a rule, their ingredients are not determinable by the unaided eye, but they are principally felspar and quartz as very minute particles. The rocks are pale-colored (yellowish or reddish as a rule), hard, splintery, much jointed, and occasionally nodular. Many felsites contain porphyritic crystals of clear quartz in rounded blebs, more or less idiomorphic felspar, and occasionally biotite. Others are entirely fine-grained and microor cryptocrystalline. Occasionally they show a fluxional banding; they may also be spherulitic or vesicular. Those which carry porphyritic quartz are known as quartz-felsites; the term soda-felsites has been applied to similar fine-grained rocks rich in soda-felspar.
Although there are few objections to the employment of felsite as a field designation for rocks having the above characters, it lacks definiteness and has been discarded by many petrologists as unsuited for the exact description of rocks, especially when their microscopic characters are taken into consideration. The felsites accordingly are broken up into "graniteporphyries," "orthophyres" and "orthoclase-porphyries," "felsitic-rhyolites," "keratophyres," "granophyres," "microgranites". But felsite or microfelsite is still the generally accepted designation for that very fine-grained, almost cryptocrystalline substance that forms the ground-mass of so many rhyolites, dacites, and porphyries.
In the hand specimen, it is a dull, lustreless, stony-looking aggregate. Under the microscope even with high powers and the very thinnest modern sections, it often cannot be resolved into its components. In places it may contain determinable minute crystals of quartz; less commonly it may show grains which can be proved to be felspar, but usually, it consists of an ultra-microscopic aggregate of fibers, threads, and grains, which react to polarized light feebly and indefinitely. Spherulitic, spotted, streaky and fluidal structures may appear in it, and many different varieties have been established on such characters as these but without much validity.
Its association with the acid rocks, its hardness, method of weathering, and chemical composition, indicate that it is an intermixture of quartz and acid felspar, and the occasional presence of these two minerals in well-defined grains confirms this. Moreover, in many dikes, while the ground-mass is microcrystalline and consists of quartz and felspar near the center of the mass, towards the margins, where it has been rapidly chilled by contact with the cold surrounding rocks, it is felsitic. The very great viscosity of acid magmas prevents their molecules, especially when cooling takes place suddenly, from arranging themselves to form discrete crystals, and is the principal cause of the production of felsitic ground-masses. In extreme cases, these conditions hinder crystallization altogether, and glassy rocks result. Some rocks are felsitic in parts but elsewhere glassy, and it is not always clear whether the felsite is an original substance or has arisen by the devitrification of primary glass. The presence of perlitic structure in some of these felsites points to the latter conclusion, and the results of an examination of ancient glasses and of artificial glass which has been slowly cooled are under this view. It has been argued that felsite is a eutectic mixture of quartz and felspar, such that when solidification takes place and the excess of felspar (or quartz) has crystallized out it remains liquid till the temperature has fallen to its freezing point, and then consolidates simultaneously. This may be so, but analyses show that it has not always the same composition and consequently that the conditions which determine its formation are not quitesimple. Felsitic rocks are sometimes silicified and have their matrix replaced by granular aggregates of cloudy quartz.
Pegmatites are extreme igneous rocks that form during the final stage of a magma’s crystallization. They are extreme because they contain exceptionally large crystals and they sometimes contain minerals that are rarely found in other types of rocks.
To be called a "pegmatite," rock should be composed almost entirely of crystals that are at least one centimeter in diameter. The name "pegmatite" has nothing to do with the mineral composition of the rock.

Fig 2.32 Topaz on albite
Most pegmatites have a composition that is similar to granite with abundant quartz, feldspar, and mica. These are sometimes called "granite pegmatites" to indicate their mineralogical composition. However, compositions such as "gabbro pegmatite," "syenite pegmatite," and any other plutonic rock name combined with "pegmatite" are possible.
Pegmatites are sometimes sources of valuable minerals such as spodumene (an ore of lithium) and beryl (an ore of beryllium) that are rarely found in economic amounts in other types of rocks. They also can be a source of gemstones. Some of the world’s best tourmaline, aquamarine, and topaz deposits have been found in pegmatites.
The Rock with Large Crystals
Large crystals in igneous rocks are usually attributed to a slow rate of crystallization. However, with pegmatites, large crystals are attributed to low-viscosity fluids that allow ions to be very mobile.
During the early stages of a magma’s crystallization, the melt usually contains a significant amount of dissolved water and other volatiles such as chlorine, fluorine, and carbon dioxide. Water is not removed from the melt during the early crystallization process, so its concentration in the melt grows as crystallization progresses. Eventually, there is an overabundance of water, and pockets of water separate from the melt.
These pockets of superheated water are extremely rich in dissolved ions. The ions in the water are much more mobile than ions in the melt. This allows them to move about freely and form crystals rapidly. This is why crystals of a pegmatite grow so large.
The extreme conditions of crystallization sometimes produce crystals that are several meters in length and weigh over one ton. For example, a large crystal of spodumene at the Etta Mine in South Dakota was 42 feet long, 5 feet in diameter, and yielded 90 tons of spodumene!

Fig 2.33 Himalaya pegmatite
Activity at the Margins of a Batholith
Pegmatites form from waters that separate from magma in the late stages of crystallization; this activity often occurs in small pockets along the margins of a batholith. Pegmatite can also form in fractures that develop on the margins of the batholith. This is how "pegmatite dikes" are formed.
Because these dikes and pockets are small in size, the mining operations that exploit them are also small. The mining of pegmatites might be done in an underground operation that follows a dike or exploits a small pocket. It can also be done at an outcrop where the pegmatite is easily discovered by people. Pegmatites usually do not support large mining operations that employ dozens of workers and have a continuous activity of many years.

Fig 2.34 Crabtree pegmatite.
Rare Minerals in Large Crystals
In the early stages of crystallization, the ions that form high-temperature minerals are depleted from the melt. Rare ions that do not participate in the crystallization of common rock-forming minerals become concentrated in the melt and the excluded water. These ions can form the rare minerals that are often found in pegmatites. Examples are small ions such as lithium and beryllium that form spodumene and beryl; or large ions such as tantalum and niobium that form minerals such as tantalite and niobite. Rare elements concentrated in large crystals make pegmatite a potential source of valuable ore.
Uses of Pegmatite
Pegmatite rock has very few uses. However, pegmatite deposits often contain gemstones, industrial minerals, and rare minerals.
Pegmatite rock has limited use as an architectural stone. Occasionally it is encountered in a dimension stone quarry that produces granite for architectural use. If the pegmatite is sound and attractive, it might be cut into slabs and polished for building facing, countertops, tile, or other decorative stone products and sold commercially as a "granite."

Fig 2.35 Polished pegmatite countertop
Some of the world’s best gemstone mines are in pegmatites. Gemstones found in pegmatite include amazonite, apatite, aquamarine, beryl, chrysoberyl, emerald, garnet, goshenite, heliodor, kunzite, lepidolite, morganite, spodumene, topaz, tourmaline, zircon, and many others. Large crystals of excellent-quality material are often found in pegmatite.
Pegmatite is the host rock for many rare mineral deposits. These minerals can be commercial sources of beryllium, bismuth, boron, cesium, lithium, molybdenum, niobium, tantalum, tin, titanium, tungsten, and many other elements. In most cases the mining operations are very small, employing less than a dozen people. If the mine contains nice crystals, the minerals are often more valuable as mineral specimens and faceting rough than being sold as an ore.
Pegmatite is often mined for industrial minerals. Large sheets of mica are mined from pegmatite. These are used to make components for electronic devices, retardation plates, circuit boards, optical filters, detector windows, and many other products. Feldspar is another mineral frequently mined from pegmatite. It is used as a primary ingredient for making glass and ceramics. It is also used as a filler in many products.
Hornfels is a fine-grained metamorphic rock that was subjected to the heat of contact metamorphism at a shallow depth. It was "baked" by heat conducted from a nearby magma chamber, sill, dike, or lava flow. Common temperatures for the formation of hornfels range from about 1300 to 1450 degrees Fahrenheit (700 to 800 degrees Celsius).
Because directed pressure does not play a significant role in the formation of hornfels, it is often made up of mineral grains that are equidimensional in shape and without a preferred orientation. The grain shape and orientation might also be inherited from its parent rock.
The name "hornfels" is assigned to rock after considering its grain size, texture, and geologic history. As a result, hornfels does not have a specific chemical or mineralogical composition. It inherits its composition from the rocks that are metamorphosed plus the fluids involved in the metamorphic process. Interpreting composition, grain size, texture, and geologic history can make hornfels a very difficult rock to identify.

Fig 2.36 Banded Hornfels
Parent Rocks and Protoliths
Hornfels is not a rock that is "deposited". Instead, it is a rock type that forms when an existing rock is metamorphosed. The original rock that was metamorphosed is usually referred to as the "parent rock" or "protolith".
A variety of sedimentary, igneous, and metamorphic rocks can be the protolith of hornfels. Common protoliths of hornfels include sedimentary rocks such as shale, siltstone, sandstone, limestone, and dolomite; igneous rocks such as basalt, gabbro, rhyolite, granite, andesite, and diabase; or, metamorphic rocks such as schist and gneiss.
Characteristics of Hornfels
Hornfels often retains the stratification, large-scale geometry, and also some textural characteristics of the protolith. The changes of contact metamorphism that convert rocks to hornfels can include recrystallization, cementation, silicification, partial melting, and more.
The result is often a dense, hard, fine-grained rock that is generally homogenous and exhibits a semi-conchoidal fracture. Hornfels can be almost any color, but black, gray, brown, reddish, and greenish rocks are common.
Based on mineral composition, most occurrences of hornfels can be separated into one of three general groups:
Pelitic Hornfels: usually derived from shale, slate, and schist
Carbonate Hornfels: usually derived from limestone, dolomite, or marble
Mafic Hornfels: usually derived from mafic igneous rocks
A wide range of minerals and mineral groups are encountered in hornfels. The minerals frequently seen includeactinolite, andalusite, augite, biotite, calcite, chlorite, cordierite, diopside, epidote, feldspars, garnet, graphite, hornblende, kyanite, pyrite, scapolite, sillimanite, sphene, tourmaline, and vesuvianite.
Amphiboles occur in contact with metamorphic aureoles around igneous intrusions. (An aureole is a zone surrounding an intrusion, which is a mass of igneous rock that solidified between other rocks located within the Earth.) The contact aureoles produced in siliceous limestones and dolomites, called skarns or calc-silicate rocks, characteristically contain metamorphic amphiboles such as tremolite or actinolite. The presence of tremolite implies a relatively low grade of metamorphism as tremolite breaks down to form the pyroxene diopside in the presence of calcite and quartz at elevated temperatures. Richterite-winchite occurs in hydrothermally metamorphosed limestones. Magnesium-rich anthophyllites are found along contact zones of granitic dikes intruding ultramafic rocks (those rich in iron and magnesium).
Regional metamorphic rocks
Many different amphiboles may be contained in regional metamorphic rocks. Commonly several amphiboles may coexist with one another in the same sample, depending on the bulk chemistry of the rock and the pressure and temperature of metamorphism. The amphiboles typically occur with plagioclase feldspar, quartz, and biotite, as well as with chlorite and oxide minerals. In magnesium-rich rocks, tremolite, anthophyllite, and hornblende may exist together. Gedrite and cummingtonite coexist with garnet in rocks enriched in aluminum and iron. Rocks containing cummingtonite or grunerite are characteristic of metamorphosed iron formations associated with iron oxides, iron-rich sheet silicates, carbonates, and quartz. Glaucophane occurs only in such metamorphic rocks as schist, eclogite, and marble. Glaucophane associated with jadeite, lawsonite, and calcite or aragonite is the characteristic assemblage found in high-pressure, low-temperature metamorphic rocks called blueschists, which have a blue color imparted by the glaucophane. Blueschists have basaltic bulk compositions and may also contain riebeckite. The latter also may occur in regional metamorphic schists. Tremolite-actinolite and sheet-silicate chlorites are the principal minerals in the low-to-moderate temperature and pressure greenschist metamorphic rocks. Hornblende is characteristic of some medium-grade metamorphic rocks known as amphibolites, in which hornblende and plagioclase are the major constituents.
Dehydration of amphiboles in the lower crust or mantle may be an important source of water that aids in the generation of magmas from partial melting processes.
Kaolinization refers to the alteration of alkali feldspar into the clay mineral kaolinite in the presence of slightly acidic solutions. Rain readily dissolves carbon dioxide (CO2) from the atmosphere, promoting weathering of granitic rocks. As demonstrated in the following reaction, in the presence of carbonic acid and water, potassium feldspar is altered to kaolinite, with potassium ion, bicarbonate, and silica in solution as byproducts.
2 KAlSi3O8 + 2 H2CO3 + 9 H2O => Al2Si2O5(OH)4 + 4 H4SiO4 + 2 K+ + 2 HCO3−
Tor is an exposed rock mass of jointed and broken blocks. Tors are seldom more than 15 meters (50 feet) high and often occur as residues at the summits of inselbergs and the highest points of pediments. Tors usually overlie unaltered bedrock and are thought to be formed either by freeze-thaw weathering or by groundwater weathering before exposure. There is often evidence of spheroidal weathering of the squared joint blocks.

Fig 2.37 Vixen Tor in Dartmoor
Granites may be defined as plutonic light-colored igneous rocks. These are among the most common igneous rocks.
The two most common and essential mineral constituents of granite are Quartz and Felspar.
Granites are generally coarse to medium-grained, holocrystalline (phaneric), and equigranular rocks. Granitic, graphic, porphyritic, and intergrowth textures are the most common types of textures met within granites of different varieties.
Types
Many types of granites are distinguished based onthe relative abundance in them of some particular accessory mineral.
Occurrence
Megasacopic Identification.
Granites may be identified in hand specimens by their:
(i) Light-coloured (leucocratic) appearance, such as grey, pink, brownish and yellowish. Some of the shades may take brilliant polish to make it eminently suitable as a decorative building stone.
(ii) Coarse to medium-grained texture; fine-grained granites are rare specimens.
(iii) Abundance of quartz and felspar orthoclase as essential minerals.
Use
Granites find extensive use in architectural and massive construction where they are found in abundance.These rocks have been used extensively in monuments and memorials, as columns and steps and as flooring, in buildings
Origin
Many minor granitic bodies occurring as sills and similar masses are clearly of igneous plutonic origin.
Definition.
These are igneous rocks of typically hypabyssal origin having formed as shallow sills and dykes. They may be regarded as equivalents of gabbros of plutonic origin and basalts of volcanic origin.
Composition.
Dolerites are predominantly made up of calcic plagioclase (e.g. anorthite and labradorite). Dark minerals like augite, olivine, and iron oxide, etc. are also present in good proportion in dolerites along with the plagioclase minerals.Dolerites are mostly medium to fine-grained rocks.Ophitic and porphyritic textures are quite common in many dolerites.
Occurrence.
Sills and dykes of doleritic composition have been recorded at many places associated with a magmatic activity. In the Singhbhum region of south Bihar, India, many doleritic dykes traverse the Singhbhum granites.
Definition
Basalts are volcanic igneous rocks formed by rapid cooling from lava flows from volcanoes either over the surface or underwater on oceanic floors. They are basic in character.
Composition.
Basalts are commonly made up of calcic plagioclase felspars (anorthite and labradorite) and many ferromagnesian minerals like augite, hornblende, hypersthene, olivine, biotite, and iron oxides, etc. Many types of basalts are distinguished based on the type and proportion of ferromagnesian minerals in them.Thus, for instance, Basanite is an olivine-rich basalt and Tepherite is an olivine-free type of basalt. The olivine-free basalts, which are quite abundant in occurrence, are sometimes named collectively as Tholeiites.
Occurrence
Basaltic rocks form when extensive lava flows on the continents and also on the oceanic floors in almost all the regions of the world. In India, the Deccan Traps, which are of basaltic and related rocks, are spread over more than four hundred thousand square kilometers in Maharashtra, Gujarat, Madhya Pradesh, and adjoining parts of the Indian Peninsula.
Gabbro
Gabbro is a coarse-grained, dark-colored, intrusive igneous rock. It is usually black or dark green and composed mainly of the minerals plagioclase and augite. It is the most abundant rock in the deep oceanic crust. Gabbro has a variety of uses in the construction industry. It is used for everything from crushed stone base materials at construction sites to polished stone countertops and floor tiles.
Minerals in Gabbro
Gabbro is composed mainly of calcium-rich plagioclase feldspar (usually labradorite or bytownite) and pyroxenes (usually augite). Minor amounts of olivine might also be present in the rock.
This mineral composition usually gives gabbro a black to very dark green color. A minor amount of light-colored mineral grains may also be present. Unlike many other igneous rocks, gabbro usually contains very little quartz. You can see a close-up view of gabbro toward the bottom of this page.
Gabbro and Basalt are Related
Gabbros are equivalent in composition to basalts. The difference between the two rock types is their grain size. Basalts are extrusive igneous rocks that cool quickly and have fine-grained crystals. Gabbros are intrusive igneous rocks that cool slowly and have coarse-grained crystals.
Gabbro in Oceanic Crust
It is often stated that Earth's oceanic crust is made up of basalt. The word "basalt" is used because the rocks of the oceanic crust have a "basaltic" composition. However, only a thin surface veneer of oceanic crust is basalt. The deeper rocks of the oceanic crust are generally coarser-grained gabbro. Basalt occurs at the surface of the crust because the rocks there have cooled quickly. At greater depth, the cooling rate is slower, and large crystals have time to develop.
Gabbro in Continental Crust
On the continents, gabbro can be found within thick lava flows of basaltic composition, where slow cooling allows large crystals to form. Gabbro will also be present in the deep plutons that form when magma chambers that feed basaltic eruptions crystallize.
Large volumes of gabbro are present beneath extensive flood basalts such as the Columbia River flood basalts of Washington and Oregon and the Deccan Traps of India.
Uses of Gabbro
Gabbro can be polished to a brilliant black luster. Brightly polished gabbro is used to make cemetery markers, kitchen countertops, floor tiles, facing stone, and other dimension stone products. It is a highly desirable rock that stands up to weathering and wear.
In the dimension stone industry, gabbro is sold under the name "black granite." Gabbro is also used to make several rough-cut products such as curbing, ashlars, paving stones, and other products.
The most common use of gabbro is as a crushed stone or aggregate. Crushed gabbro is used as a base material in construction projects, as a crushed stone for road construction, as railroad ballast, and anywhere that a durable crushed stone is needed as fill.
Gabbro as an Ore
Gabbro sometimes contains economic amounts of some relatively rare metals. Gabbros containing significant amounts of the mineral ilmenite are mined for their titanium content. Other gabbros are mined to yield nickel, chromium, or platinum.
Basalt is the most common rock on Earth’s surface. Specimens are black in color and weather to dark green or brown. Basalt is rich in iron and magnesium and is mainly composed of olivine, pyroxene, and plagioclase. Most specimens are compact, fine-grained, and glassy. They can also be porphyritic, with phenocrysts of olivine, augite, or plagioclase. Holes left by gas bubbles can give basalt a coarsely porous texture.
Group – volcanic.
Colour –dark grey to black.
Texture – aphanitic (can be porphyritic).
Mineral content – groundmass generally of pyroxene ( augite), plagioclase, and olivine, possibly with minor glass; if porphyritic the phenocrysts will be any of olivine, pyroxene or plagioclase.
Silica (SiO 2) content – 45%-52%.

Fig 2.38 Basalt Rock
Basalt makes up large parts of the ocean floor. It can form volcanic islands when it is erupted by volcanoes in ocean basins. The rock has also built
huge plateaus on land. The dark plains on the Moon, known as maria, and, possibly, the volcanoes on Mars and Venus are made of basalt.

Fig 2.39 Vesicular and Amygdaloidal Textures
Basalt has a strict chemical definition. It is defined in the TAS diagram shown above. Basalt is an igneous rock that contains more than 45 and less than 52% of SiO2 and less than five percent of total alkalies (K2O + Na2O)3.
Classification
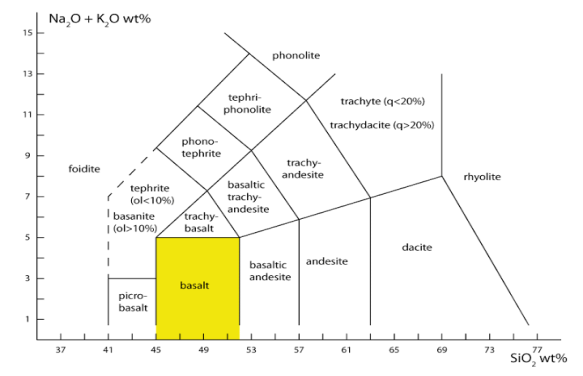
Fig 2.40 TAS diagram
Types of Basalt
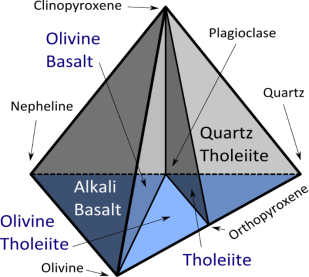
Fig 2.41 Types of basalt
Basalt types: tholeiites vs alkali basalts
Tholeiitic basalt is relatively rich in silica and poor in sodium. Included in this category are most basalts of the ocean floor, most large oceanic islands, and continental flood basalts such as the Columbia River Plateau.
High and low titanium basalts.
Basalt rocks are in some cases classified after their titanium (Ti) content in High-Ti and Low-Ti varieties. High-Ti and Low-Ti basalts have been distinguished in the Paraná and Etendeka traps and the Emeishan Traps.
Mid-ocean ridge basalt (MORB) is a tholeiitic basalt commonly erupted only at ocean ridges and is characteristically low in incompatible elements
High-alumina basaltmay be silica-undersaturated or -oversaturated (see normative mineralogy). It has greater than 17% alumina (Al2O3) and is intermediate in composition between tholeiitic basalt and alkali basalt; the relatively alumina-rich composition is based on rocks without phenocrysts of plagioclase.
Alkali basalt is relatively poor in silica and rich in sodium. It is silica-undersaturated and may contain feldspathoids, alkali feldspar, and phlogopite.

Fig 2.42 Alkaline Basalt
Boninite is a high-magnesium form of basalt that is erupted generally in back-arc basins, distinguished by its low titanium content and trace-element composition.
Petrology
The mineralogy of basalt is characterized by a preponderance of calcic plagioclase feldspar and pyroxene. Olivine can also be a significant constituent. Accessory minerals present in relatively minor amounts include iron oxides and iron-titanium oxides, such as magnetite, ulvospinel, and ilmenite. Because of the presence of such oxide minerals, basalt can acquire strong magnetic signatures as it cools, and paleomagnetic studies have made extensive use of basalt.
Columnar basalt
During the cooling of a thick lava flow, contractional joints or fractures form. If a flow cools relatively rapidly, significant contraction forces build up. While a flow can shrink in the vertical dimension without fracturing, it can’t easily accommodate shrinking in the horizontal direction unless cracks form; the extensive fracture network that develops results in the formation of columns. The topology of the lateral shapes of these columns can broadly be classed as a random cellular network. These structures are predominantly hexagonal in cross-section, but polygons with three to twelve or more sides can be observed.The size of the columns depends loosely on the rate of cooling; very rapid cooling may result in very small (<1 cm diameter) columns, while slow cooling is more likely to produce large columns.

Fig 2.43 Columnar Basalt
Pillow basalts
When basalt erupts underwater or flows into the sea, contact with the water quenches the surface and the lava forms a distinctive pillowshape, through which the hot lava breaks to form another pillow. This “pillow” texture is very common in underwater basaltic flows and is diagnostic of an underwater eruption environment when found in ancient rocks. Pillows typically consist of a fine-grained core with a glassy crust and have radial jointing. The size of individual pillows varies from 10 cm up to several meters.

Fig 2.44 Pillow basalt at Point Bonita
Alteration
Metamorphism
Basalts are important rocks within metamorphic belts, as they can provide vital information on the conditions of metamorphism within the belt.
Metamorphosed basalts are important hosts for a variety of hydrothermal ore deposits,including gold deposits, copper deposits, volcanogenic massive sulfide ore deposits, and others.
Weathering
Compared to other rocks found on Earth’s surface, basalts weather relatively fast. The typically iron-rich minerals oxidize rapidly in water and air, staining the rock a brown to red color due to iron oxide (rust). Chemical weathering also releases readily water-soluble cations such as calcium, sodium, and magnesium, which give basaltic areas a strong buffer capacity against acidification. Calcium released by basalts binds up CO2 from the atmosphere forming CaCO3 acting thus as a CO2 trap. To this, it must be added that the eruption of basalt itself is often associated with the release of large quantities of CO2 into the atmosphere from volcanic gases.
Uses of Basalt
Basalt is used in construction (e.g. as building blocks or in the groundwork), making cobblestones (from columnar basalt) and in making statues. Heating and extruding basalt yields stone wool said to be an excellent thermal insulator.
Carbon sequestration in basalt has been studied as a means of removing carbon dioxide, produced by human industrialization, from the atmosphere. Underwater basalt deposits, scattered in seas around the globe, have the added benefit of the water serving as a barrier to the re-release of CO2 into the atmosphere.
Sedimentary rock, rock formed at or near Earth’s surface by the accumulation and lithification of sediment (detrital rock) or by the precipitation from solution at normal surface temperatures (chemical rock). Sedimentary rocks are the most common rocks exposed on Earth’s surface but are only a minor constituent of the entire crust, which is dominated by igneous and metamorphic rocks.
Mode of formation
Sedimentary rocks are produced by the weathering of preexisting rocks and the subsequent transportation and deposition of the weathering products. Weathering refers to the various processes of physical disintegration and chemical decomposition that occur when rocks at Earth’s surface are exposed to the atmosphere (mainly in the form of rainfall) and the hydrosphere. These processes produce soil, unconsolidated rock detritus, and components dissolved in groundwater and runoff. Erosion is the process by which weathering products are transported away from the weathering site, either as solid material or as dissolved components, eventually to be deposited as sediment. Any unconsolidated deposit of solid weathered material constitutes sediment. It can form as the result of deposition of grains from moving bodies of water or wind, from the melting of glacial ice, and from the downslope slumping (sliding) of rock and soil masses in response to gravity, as well as by precipitation of the dissolved products of weathering under the conditions of low temperature and pressure that prevail at or near the surface of Earth.
Sedimentary rocks are the lithified equivalents of sediments. They typically are produced by cementing, compacting, and otherwise solidifying preexisting unconsolidated sediments. Some varieties of sedimentary rock, however, are precipitated directly into their solid sedimentary form and exhibit no intervening existence as sediment. Organic reefs and bedded evaporites are examples of such rocks. Because the processes of physical (mechanical) weathering and chemical weathering are significantly different, they generate markedly distinct products and two fundamentally different kinds of sediment and sedimentary rock: (1) terrigenous clastic sedimentary rocks and (2) allochemical and orthochemical sedimentary rocks.
Mineralogical Composition
Because of their detrital nature, any mineral can occur in sedimentary rock. Clay minerals, the dominant mineral produced by the chemical weathering of rocks, are the most abundant mineral in mudrocks. Quartz, because it is stable under conditions present at the surface of the Earth, and because it is also a product of chemical weathering, is the most abundant mineral in sandstones and the second most abundant mineral in mudrocks. Feldspar is the most common mineral in igneous and metamorphic rocks. Although feldspar eventually breaks down to clay minerals and quartz, it is still the third most abundant mineral in sedimentary rocks. Carbonate minerals, either precipitated directly or by organisms, make up most biochemical and chemical sedimentary rocks, but carbonates are also common in mudrocks and sandstones.
Mineral Composition | Mudrocks % | Sandstones % |
Clay minerals | 60 | 5 |
Quartz | 30 | 65 |
Feldspar | 4 | 10 - 15 |
Carbonate minerals | 3 | <1 |
Organic matter, hematite, & others | <3 | <1 |
Table 2.3
Minerals found in sedimentary rocks can be divided into 2 classes:
Any mineral can be an allogenic mineral, but some are more stable under the conditions present at the Earth's surface than are others. Conditions that are present at the Earth's surface and differ from those where most minerals form are:
Because these conditions differ from those under which most rocks form, allogenic minerals can be classified based on their stability under near-surface conditions. Such a classification, with minerals listed in order of increasing stability, is as follows:
Stability Under Conditions Present at Surface | Mineral |
Unstable | Olivines* |
Pyroxenes* | |
Ca-rich Plagiocalse* | |
Hornblende* | |
Andesine - Oligoclase* | |
Less Unstable | Sphene |
Epidote | |
Andalusite | |
Staurolite | |
Kyanite | |
Sillimanite | |
Magnetite | |
Garnet | |
Very Stable | Muscovite* |
Albite* | |
Orthoclase/Microcline* | |
Clay Minerals | |
Quartz* | |
Tourmaline | |
Zircon |
Table 2.4
In this list, the igneous minerals have an asterisk (*). Note that the order in which they occur is in the same order that occurs in Bowen's reaction series. Igneous minerals that crystallize at the highest temperatures are most out of equilibrium at the Earth's surface and are therefore the most unstable.
Minerals that are very stable at the Earth's surface are minerals that either form as a result of chemical weathering, or crystallize at the lowest temperatures.
Authigenic minerals can also be allogenic minerals, but some are formed during diagenesis but not very stable in the transportation cycle either because they dissolve readily in water or because they are easily abraded during transportation. Thus we can divide authigenic minerals into those that are stable during diagenesis and transportation, and those that are unstable during transportation.
Unstable Authigenic Minerals | Gypsum |
Carbonate Minerals | |
Apatite | |
Glauconite | |
Pyrite | |
Zeolites | |
Stable Authigenic Minerals | Chlorite |
Albite | |
Orthoclase | |
Muscovite | |
Quartz | |
Clay Minerals |
Table 2.5
The longer a mineral is in the weathering and transportation cycles of sedimentary rock-forming processes, the more likely it is to break down to a more stable mineral or disappear altogether. Thus, we can classify sediments based on which they have achieved mineralogical maturity.
Textures of Sedimentary Rocks
Since most sedimentary rocks are derived by processes of weathering, transportation, deposition, and diagenesis, the textures we find in sediment and sedimentary rocks are dependent on a process that occur during each stage. These include:
2. The strength of the wind or water currents that carry and deposit the sediment. This determines whether or not grains are transported or deposited. The deposition process also controls structures that could be preserved in the sediment and thus give clues to the environment of deposition.
3. The distance transported or time in the transportation process. The longer grains are in the transportation process the more likely they are to change shape and become sorted based on size and mineralogy. This also controls the extent to which they break down to stable minerals during the transportation process.
4. Biological activity with the sediment before diagenesis. Burrowing organisms can redistribute sediment after it has been deposited, thus erasing some of the clues to the original environment of deposition.
5. The chemical environment under which diagenesis occurs. During diagenesis grains are compacted, new minerals precipitate in the pore spaces, some minerals continue to react to produce new minerals, and some minerals recrystallize. What happens depends on the composition of fluids moving through the rock, the composition of the mineral grains, and the pressure and temperature conditions attained during diagenesis.
Grain Size
Clastic sediments and sedimentary rocks are classified based on the predominant grain size of clasts in the rock as per the table below.
Name of Particle | Size Range | f Scale | Loose Sediment | Consolidated Rock |
Boulder | >256 mm | <-8 |
Gravel
| Conglomerate or Breccia |
Cobble | 64 - 256 mm | -6 to -8 | ||
Pebble | 4 - 64 mm | -2 to -6 | ||
Granule | 2 - 4 mm | -1 to -2 | ||
Very Coarse Sand | 1 - 2 mm | 0 to -1 | Sand | Sandstone |
Coarse Sand | 0.5 - 1 mm | 1 to 0 | ||
Medium Sand | 0.25 - 0.5 mm | 2 to 1 | ||
Fine Sand | 0.125 - 0.25 mm | 3 to 2 | ||
Very Fine Sand | 0.0625 - 0.125 mm | 4 to 3 | ||
Coarse Silt | 0.031 - 0.625 mm | 5 to 4 |
Silt
| Siltstone |
Medium Silt | 0.016 - 0.031 mm | 6 to 5 | ||
Fine Silt | 0.008 - 0.016 mm | 7 to 6 | ||
Very Fine Silt | 0.004 - 0.008 mm | 8 to 7 | ||
Clay | <0.004 mm | >8 | Clay | Claystone, Mudstone, Shale |
Table 2.6
Note how the particle sizes small than pebble size is defined. The lower size limit of granules is 1/2 the lower size limit of pebbles, the lower size limit of coarse sand is 1/2 the lower size limit of granules, etc. For this reason, grain size is often given in units called f (phi) units, where f is defined as follows:
f = -log2(d)
where d is the grain diameter in millimeters.
This is convenient for grain size analyses of sediment, or rocks if they can be disaggregated because sieves can be constructed where the opening of each sieve is 1/2 the size of the sieve above. Sediment put through such a set of sieves, will be trapped in different sieves, and sorted by grain size. The number of grains in each sieve can then be weighed to give a quantitative measure of the size distribution of sediment.
One precaution- note how the large grain sizes have negative f units.
Sorting - Sorting refers to the uniformity of grain size in a sediment or sedimentary rock. Particles become sorted based on density because of the energy of the transporting medium. High energy (high velocity) currents can carry larger fragments.
As the energy or velocity decreases, heavier particles are deposited and lighter fragments continue to be transported. This results in sorting due to density. If the particles have the same density, such as all grains of quartz, then the heavier particles will also be larger, so the sorting will take place based on size. We can classify this size sorting on a relative basis: well-sorted to poorly-sorted.
In a more quantitative sense, sediment can be sieved to determine the distribution of grain size, as noted above.By weighing the amount of sediment or disaggregated sedimentary rock that occurs in each sieve then re-summing to 100%, a histogram can be constructed to show the size distribution of the sediment. To simplify matters, we can also construct a frequency curve by drawing a smooth curve through the midpoints of the histogram.

Fig 2.45
In a quantitative sense, sorting is a measure of the standard deviation from the mean grain size. Thus, on a histogram or frequency curve, a narrow peak has a low standard deviation and would represent well-sorted material. A broad, spread-out frequency curve would indicate a poorly sorted sediment.
Beach sands and dune sands tend to be well-sorted because the energy of the waves or wind is usually rather constant. The coarser-grained sediment is not carried in because the wave or wind velocity is too low to carry such large fragments, and the finer-grained sediment is kept in suspension by the waves or wind.
Mountain streams, because they have many turbulent eddies where the velocity of the stream changes suddenly usually show poorly-sorted sediment on the bottom of the stream channel. Similarly, glacial till, because it is deposited in place as glacial ice melts, and is not transported by water, tends to show poor sorting.
Rounding - During the transportation process, grains may be reduced in size due to abrasion. Random abrasion results in the eventual rounding off of the sharp corners and edges of grains. Thus, the degree of rounding of grains gives us clues to the amount of time sediment has been in the transportation cycle. Rounding is classified on relative terms as well. Note that rounding is not the same asphericity. Sphericity is controlled by the original shape of the grain.
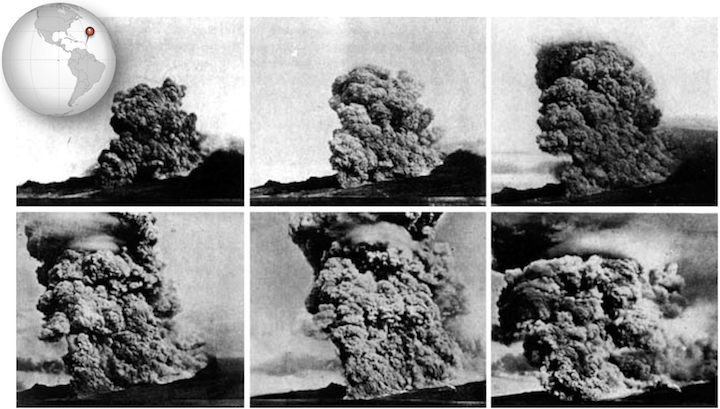
Fig 2.46
Porosity- Porosity is the percentage of the volume of the rock that is open space (pore space). This determines the amount of water or other fluids, like petroleum, that a rock can contain.

Fig 2.47
In sediments or sedimentary rocks, the porosity depends on grain size, the shapes of the grains, the degree of sorting, and the degree of cementation.
Well-rounded coarse-grained sediments usually have higher porosity than fine-grained sediments, because the grains do not fit together well. Angular grains of fine-grained sediment can be compacted to fit together better, and thus porosity is reduced. Mudrocks, because of fine grain size, usually have very low porosities.
Poorly sorted sediments usually have lower porosity because the fine-grained fragments tend to fill in the open space
Cementation that takes place during diagenesis tends to fill in the pore space. Thus, highly cemented sedimentary rocks have lower porosity than do poorly cemented sedimentary rocks.

Fig 2.48
Packing of Grains- Packing refers to the arrangement of clastic grains entirely apart from any authigenic cement that may have later crystallized between them. If the clastic grains touch each other throughout, the rock is said to be grain supported. If the rock is poorly sorted and the grains are separated by a mud or silt matrix, the rock is said to be matrix supported.
Matrix- If the rock is poorly or moderately sorted, the percentage of matrix and texture of the matrix should also be described.
Induration- Induration refers to the hardness of the rock or how easily it breaks apart. Wellindurated rocks are difficult to break with a hammer. Moderately indurated rocks can be easily broken with a rock hammer. Poorly indurated or friable rocks break apart easily in your hand. The term non-indurated would describe sediment that has not undergone any cementation.
Textural MaturityThe longer sediment is involved in the transportation cycle, the more time it has to become well-sorted. Similarly, the longer the sediment is transported, the more time is available for grains to lose their rough edges and corners by abrasion. Thus, we consider texturally mature sediment to be sediment that is well-sorted and well-rounded. Note that sediment tends to become both texturally and mineralogically mature the longer it is in the transportation cycle.
Descriptions of TextureA complete description of the texture of a sedimentary rock should include statements about each of the factors discussed above. To summarize, these are:
2 Size of the grains.
3 Sorting.
4 Degree of roundness and sphericity of the grains.
5 An estimate of the porosity of the rock.
6 Packing of the grains.
7 A description of the matrix.
8 Induration of the rock.
9 A statement about the textural maturity of the rock.
Structures of Sedimentary RocksThe process of deposition usually imparts variations in layering, bedforms, or other structures that give clues to the environment in which deposition occurs. Such things as water depth, current velocity, and current direction can be sometimes be determined from sedimentary structures. Thus, it is important to recognize various sedimentary structures so that we can interpret the clues that they offer to these conditions.
Also, because sedimentary rocks can be deformed by folding and faulting long after the deposition process has ended, it is important to be able to determine which was up in the rock when it was originally deposited, especially if one is going to use the rocks to determine the sequence of events that occurred or interpret the geologic history of an area. Features that tell us which way was up are often referred to as top and bottom indicators.
One of the most obvious features of sedimentary rocks and sediment is the layered structure which they exhibit. The layers are evident because of differences in mineralogy, clast size, degree of sorting, or color of the different layers. In rocks, these differences may be made more prominent by the differences in resistance to weathering or color changes brought out by weathering.
Layering is usually described based on layer thickness as per the table below. Other distinctive types of layering are described below.
Layer Thickness | Names |
> 300 cm | Massive |
100-300 cm | Very thickly bedded |
30 - 100 cm | Thickly bedded |
10 - 30 cm | Mediumly bedded |
3 - 10 cm | Thinly bedded |
1 - 3 cm | Very thinly bedded |
0.3 - 1 cm | Thickly laminated |
<0.3 cm | Thinly laminated |
Table 2.7
Rhythmic Layering - consists of alternating parallel layers having different properties.
Fig 2.49 Rhythmic layering
This is sometimes caused by seasonal changes in deposition (Varves). i.e. lake deposits wherein coarse sediment is deposited in summer months and fine sediment is deposited in the winter when the surface of the lake is frozen.
Cross Bedding - consists of sets of beds that are inclined relative to one another. The beds are inclined in the direction that the wind or water was moving at the time of deposition. Boundaries between sets of cross-beds usually represent an erosional surface. Cross bedding is very common in beach deposits, dunes, and river deposited sediment. Individual beds within cross-bedded strata are useful indicators of current direction and tops and bottoms. Note how the beds become asymptotic to the lower boundary on which they were deposited.
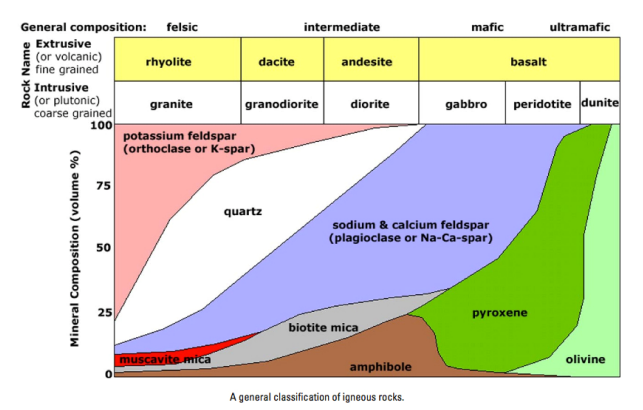
Fig 2.50 Cross bedding
Graded Bedding - As current velocity decreases, the larger or more dense particles are deposited first, followed by smaller particles. This results in bedding showing a decrease in grain size from the bottom of the bed to the top of the bed. This gives us a method for determining tops and bottoms of beds, since reverse grading will not be expected unless deposition occurs under unusual circumstances. Note that reverse graded bedding cannot occur as current velocity increases, because each layer will simply be removed as the current achieves a velocity high enough to carry sediment of a particular size.
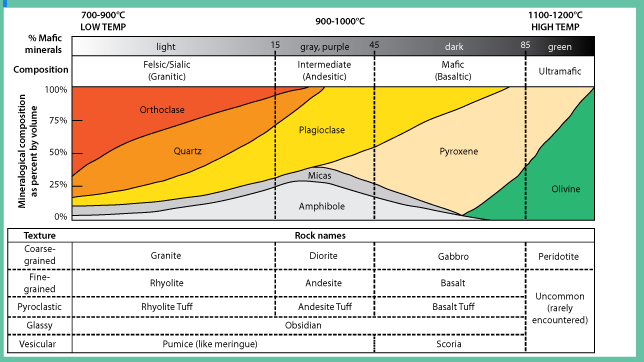
Fig 2.51 Graded bedding
Non-sorted Sediment - Sediment showing a mixture of grain sizes results from such things as rockfalls, debris flows, mudflows, and deposition from melting ice.
Imbricate bedding - Elongated grains can sometimes pile up on each other to form what is called imbricate bedding. Note that imbricate bedding can be a current direction indicator if some other means is present to provide top/bottom directions.
Surface Features
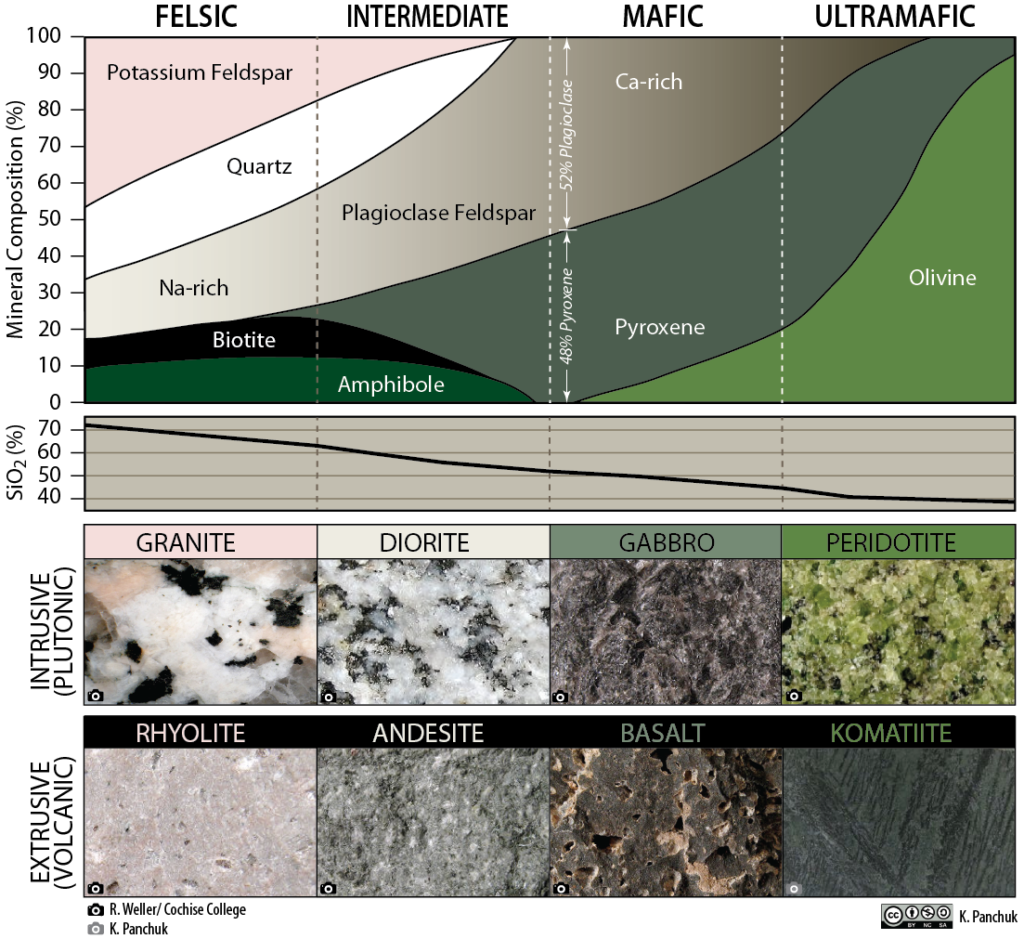
Fig 2.52 Ripple marks
Symmetrical ripple marks occur in environments where there are a steady back and forth movement of the water. Such ripple marks can still be used as top and bottom indicators.
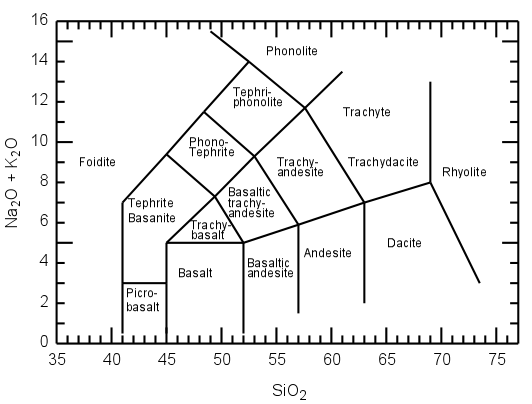
Fig 2.53 Mudcracks
Fig 2.54 Burrow marks
Siliciclastic sedimentary rocks are composed of mainly silicate particles derived from the weathering of older rocks and pyroclastic volcanism. While grain size, clast and cementing material (matrix) composition, and texture are important factors when regarding composition, siliciclastic sedimentary rocks are classified according to grain size into three major categories: conglomerates, sandstones, and mudrocks. The term clay is used to classify particles smaller than .0039 millimeters. However, the term can also be used to refer to a family of sheet silicate minerals.[3] Silt refers to particles that have a diameter between .062 and .0039 millimeters. The term mud is used when clay and silt particles are mixed in the sediment; mudrock is the name of the rock created with these sediments. Furthermore, particles that reach diameters between .062 and 2 millimeters fall into the category of sand. When sand is cemented together and lithified it becomes known as sandstone. Any particle that is larger than two millimeters is considered gravel. This category includes pebbles, cobbles, and boulders. Like sandstone, when gravels are lithified they are considered conglomerates.
Conglomerates and breccias
Fig 2.55 Conglomerate
Fig 2.56 Breccia. Notice the angular nature of the large clasts
Conglomerates are coarse-grained rocks dominantly composed of gravel-sized particles that are typically held together by a finer-grained matrix.[4] These rocks are often subdivided into conglomerates and breccias. The major characteristic that divides these two categories is the amount of rounding. The gravel-sized particles that makeup conglomerates are well rounded while in breccias they are angular. Conglomerates are common in stratigraphic successions of most, if not all, ages but only make up one percent or less, by weight, of the total sedimentary rock mass. In terms of origin and depositional mechanisms, they are very similar to sandstones. As a result, the two categories often contain the same sedimentary structures.
Sandstones
Fig 2.57 Sandstone from Lower Antelope Canyon
Sandstones are medium-grained rocks composed of rounded or angular fragments of sand size, that often but not always have cement uniting them together. These sand-size particles are often quartz but there are a few common categories and a wide variety of classification schemes that classify sandstones based on composition. Classification schemes vary widely, but most geologists have adopted the Dott scheme which uses the relative abundance of quartz, feldspar, and lithic framework grains and the abundance of muddy matrix between these larger grains.
MudrocksRocks that are classified as mudrocks are very fine-grained. Silt and clay represent at least 50% of the material that mudrocks are composed of. Classification schemes for mudrocks tend to vary, but most are based on the grain size of the major constituents. In mudrocks, these are generally silt and clay.
According to Blatt, Middleton, and Murray mudrocks that are composed mainly of silt particles are classified as siltstones. In turn, rocks that possess clay as the majority particle are called claystone. In geology, a mixture of both silt and clay is called mud. Rocks that possess large amounts of both clay and silt are called mudstones. In some cases, the term shale is also used to refer to mudrocks and is still widely accepted by most. However, others have used the term shale to further divide mudrocks based on the percentage of clay constituents. The plate-like shape of clay allows its particles to stack on top of one another, creating laminae or beds. The more clay present in a given specimen, the more laminated a rock is. Shale, in this case, is reserved for mudrocks that are laminated, while mudstone refers to those that are not.
Rivers, oceans, winds, and rain runoff all can carry the particles washed off of eroding rocks. Such material, called detritus, consists of fragments of rocks and minerals. When the energy of the transporting current is not strong enough to carry these particles, the particles drop out in the process of sedimentation. This type of sedimentary deposition is referred to as clastic sedimentation. Another type of sedimentary deposition occurs when the material is dissolved in water, and chemically precipitates from the water. This type of sedimentation is referred to as chemical sedimentation. A third process can occur, wherein living organisms extract ions dissolved in water to make such things as shells and bones. This type of sedimentation is called biochemical sedimentation. The accumulation of plant matter, such as at the bottom of a swamp, is referred to as organic sedimentation. Thus, there are 4 major types of sedimentary rocks: Clastic Sedimentary Rocks, Chemical Sedimentary Rocks, Biochemical Sedimentary Rocks, and Organic Sedimentary Rocks.
Clastic Sediments and Sedimentary Rocks
The formation of clastic sediment and sedimentary rocks involves five processes:
Classification
Clastic sedimentary particles and sedimentary rocks are classified in terms of grain size and shape, among other factors.
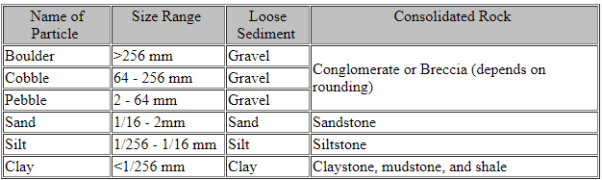
Table 2.8
In general, the coarser sediment gets left behind by the transportation process. Thus, coarse sediment is usually found closer to its source and fine-grained sediment is found farther from the source.
Types of Clastic Sedimentary Rocks
We next look at various clastic sedimentary rocks that result from the lithification of sediment.
Conglomerates and Breccias
Conglomerate and Breccia are rocks that contain an abundance of coarse-grained clasts (pebbles, cobbles, or boulders
Sandstones
A Sandstone is made of sand-sized particles and forms in many different depositional settings.
Mudrocks
Mudrocks are made of fine-grained clasts (silt and clay-sized). Siltstone is one variety that consists of silt-sized fragments.
Biochemical and Organic Sediments and Sedimentary Rocks
Biochemical and Organic sediments and sedimentary rocks are those derived from living organisms. When the organism dies, the remains can accumulate to become sediment or sedimentary rock. Among the types of rock produced by this process are:
Biochemical Limestone - calcite (CaCO3) is precipitated by organisms usually to form a shell or other skeletal structure. Accumulation of these skeletal remains results in limestone. Sometimes the fossilized remains of the organism are preserved in the rock, other times recrystallization during lithification has destroyed the remains. Limestones are very common sedimentary rocks.
Biochemical Chert - Tiny silica secreting planktonic organisms like Radiolaria and Diatoms can accumulate on the seafloor and recrystallize during lithification to form biochemical chert. The recrystallization results in a hard rock that is usually seen as thin beds (see figure 7.8a in your test).
Diatomite - When diatoms accumulate and do not undergo recrystallization, they form a white rock called diatomite as seen in the White Cliffs of Dover (see figure 7.22b in your text).
Coal - Coal is an organic rock made from organic carbon that is the remains of fossil plant matter. It accumulates in lush tropical wetland settings and requires deposition in absence of Oxygen. It is high in carbon and can easily be burned to obtain energy.
Chemical Sediments and Sedimentary Rocks
Dissolved ions released into the water by the weathering process are carried in streams or groundwater. Eventually, these dissolved ions end up in the ocean, explaining why seawater is salty. When water evaporates or the concentration of the ions gets too high as a result of some other process, the ions recombine by chemical precipitation to form minerals that can accumulate to become chemical sediments and chemical sedimentary rocks. Among these are:
Evaporites - formed by evaporation of seawater or lake water. Produces halite (salt) and gypsum deposits by chemical precipitation as the concentration of solids increases due to water loss by evaporation. This can occur in lakes that have no outlets (like the Great Salt Lake) or restricted ocean basins like has happened in the Mediterranean Sea or the Gulf of Mexico in the past.
Travertine - Groundwater containing dissolve Calcium and bicarbonate ions can precipitate calcite to form a chemically precipitated limestone, called travertine. This can occur in lakes, hot springs, and caves.
Dolostones - Limestone that has been chemically modified by Mg-rich fluids flowing through the rock are converted to dolostones. CaCO3 is recrystallized to a new mineral dolomite CaMg(CO3)2.
Chemical Cherts - Groundwater flowing through rock can precipitate SiO2 to replace minerals that were present. This produces a non-biogenic chert. There are many varsities of such chert that are given different names depending on their attributes, For example:
Flint – Black or gray from organic matter.
Jasper – Red or yellow from Fe oxides.
Petrified wood – Wood grain preserved by silica.
Agate – Concentrically layered rings
A conglomerate is a clastic sedimentary rock made up of rounded clasts that are greater than two millimeters in diameter. The spaces between the clasts are generally filled with sand- and clay-size particles. The rock is bound together by a cement that is usually composed of calcite or quartz.
Fig 2.58 Conglomerate Close-Up
Composition
A conglomerate can have a variety of compositions. As a clastic sedimentary rock, it can contain clasts of any rock material or weathering product that is washed downstream or down current.
The rounded clasts of conglomerate can be mineral particles such as quartz or feldspar, or they can be sedimentary, metamorphic, or igneous rock fragments. Clastsof quartzite, sandstone, limestone, granite, basalt, and gneiss are especially common.
The matrix that binds the clasts together can be a mixture of sand, mud, and chemical cement. Common chemical types of cement are calcite or quartz.
Fig 2.59 Conglomerate-Forming Environment

Fig 2.60 Conglomerate-Size Sediment Clasts
Formation
Conglomerate forms where sediments of rounded clasts at least two millimeters in diameter accumulate. It takes a strong water current to transport and produce a rounded shape on particles this large. Wind transport is unlikely to produce a conglomerate.
The environment of deposition might be along a swiftly flowing stream or a beach with strong waves. These conditions might only be met during times of extreme flow or wave action. However, it is during these times that much of the Earth's sediments are picked up, moved, and deposited.
To form a conglomerate, there must also be a source of large-size sediment particles somewhere up the current. The rounded shape of the clasts reveals that they were tumbled for some distance by running water or moving waves. These conditions are found in streams, lakes, and oceans in many parts of the world.
Conglomerates often begin when sediment consisting mainly of pebble- and cobble-size clasts are being deposited. The finer-size sand and clay, which fill the spaces between the larger clasts, is often deposited later on top of the large clasts and then sifts down between them to fill the interstitial spaces. After compaction, the precipitation of a chemical cement between the grains will bind the sediment into a rock.
Uses of Conglomerate
The conglomerate has very few commercial uses. Its inability to break cleanly makes it a poor candidate for dimension stone, and its variable composition makes it a rock of unreliable physical strength and durability.
The conglomerate can be crushed to make a fine aggregate that can be used where a low-performance material is suitable. Many conglomerates are colorful and attractive rocks, but they are rarely used as ornamental stones.
Analysis of conglomerate can sometimes be used as a prospecting tool. For example, most diamond deposits are hosted in kimberlite. If a conglomerate contains clasts of kimberlite, then the source of that kimberlite must be upstream of the location where the kimberlite clast was deposited. That sounds simple, but the kimberlite clast might have been deposited a few million years ago in a different landscape - but people have been successful in using this type of clue to successfully locate a diamond deposit.
In rare instances, the conglomerate can be a "fossil placer deposit" containing gold, diamonds, or other valuable minerals. These conglomerates are mined, crushed, and processed as ores.
Puddingstone is a nonscientific name for a conglomerate made up of clasts that contrast sharply with the color of the rock's matrix. People in what is today the United Kingdom were the first to use the name. They claim that the rocks reminded them of a "plum pudding". Puddingstones are found in many parts of the world. We have an article with images of puddingstones from France, Canada, India, Maryland (USA), the United Kingdom, and Mars.

Fig 2.61 Puddingstone
Conglomerate on Mars
In September 2012, NASA's Mars rover Curiosity discovered an outcrop of conglomerate exposed on the surface of Mars. The rounded clasts within the conglomerate provide evidence that a stream or a beach had moved the rocks and tumbled them into rounded pebbles. This conglomerate is one of the most convincing pieces of evidence that water once flowed on the surface of Mars.

Fig 2.62 Martian Conglomerate
Breccia is a term most often used for clastic sedimentary rocks that are composed of large angular fragments (over two millimeters in diameter). The spaces between the large angular fragments are filled with a matrix of smaller particles and a mineral cement that binds the rock together.

Fig 2.63 Debris Flow Breccia
Formation
Breccia forms where broken, angular fragments of rock or mineral debris accumulate. One of the most common locations for breccia formation is at the base of an outcrop where mechanical weathering debris accumulates. Another is in-stream deposits a short distance from the outcrop or on an alluvial fan.
Some breccias form from debris-flow deposits. The angular particle shape reveals that they have not been transported very far (transport wears the sharp points and edges of angular particles into rounded shapes). After deposition, the fragments are bound together by a mineral cement or by a matrix of smaller particles that fills the spaces between the fragments.
In arid and semiarid areas, the precipitation of mineral cement in shallow sediments or soils can result in the formation of extensive rock units known as "caliche." These materials often have the appearance of breccia and fit the definition.

Fig 2.64 Limestone Breccia
Composition
Breccia has many compositions. Its composition is mainly determined by the rock and mineral material that the angular fragments were produced from. The climate of the source area can also influence the composition. Most breccias are a mix of rock fragments and mineral grains.
The type of rock that the fragments were produced from is often used as an adjective when referring to the rock. Some examples: sandstone breccia, limestone breccia, granite breccia, chert breccia, basalt breccia, and others. Often a breccia will contain many types of angular rock fragments. These are known as polymict breccias or polymictic breccias.
Color
Breccia can be any color. The color of the matrix or cement along with the color of the angular rock fragments determines its color. Breccia can be a colorful rock

Fig 2.65 Impact Breccia
Other Ways of using Breccia
Geologists have been very generous in their use of the word "breccia." It is common to hear the term used when referring to a rock or rock debris made up of angular fragments. Although it is mainly used for rocks of sedimentary origin, it can be used for other types of rocks. A few more uses of the word are given below.
Collapse Breccia: Broken rock that originates from a cavern or magma chamber collapse.
Fault Breccia or Tectonic Breccia: Broken rock found in the contact area between two fault blocks and produced by the movement of the fault.
Flow Breccia: A lava texture produced when the crust of a lava flow is broken and jumbled during movement.
Fold Breccia: A breccia formed by the folding and breakage of thin, brittle rock layers which are interlayered with incompetent, ductile layers.
Igneous Breccia or Volcanic Breccia: A term used for a rock composed of angular fragments of igneous rocks. "Flow breccia" and "pyroclastic breccia" could be called "igneous breccia."
Impact Breccia: A deposit of angular rock debris produced by the impact of an asteroid or other cosmic body. See an article about "impactites."
Monomict Breccia: A breccia whose clasts are composed of a single rock type, possibly all from a single rock unit.
Polymict Breccia: A breccia whose clasts are composed of many different rock types.
Pyroclastic Breccia: A term used for a deposit of igneous rock debris that was ejected by a volcanic blast or pyroclastic flow.
When you hear the word "breccia" used about a rock or rock material, it is fairly safe to assume that it means angular-shaped pieces.

Fig 2.66 Breccia as a Gemstone
Uses of Breccia
The rock, breccia, has very few uses. It can be used as a fill or road base where the technical requirements are minimal. It is rarely used in important projects because its composition, degree of cementation, and competence are highly variable.
The word "breccia" is used as a trading name for a group of dimension stone products with a broken, angular pattern. Names such as "Breccia Oniciata," "Breccia Pernice," and "Breccia Damascata" are cut and polished limestones and marbles that reveal a broken, angular pattern. These breccias are used as architectural stones for interior building veneers, tiles, window sills, and other decorative applications. These are proprietary names applied to the rock from specific quarries.
Sandstones make up only about 25% of the stratigraphic record but have received the most attention in studies of sedimentary rocks. There are two reasons for this. First, sandstones are easily studied because they contain sand-sized grains that can easily be distinguished with a petrographic microscope. Second, most of the world's oil and natural gas are found in sands or sandstones because of their generally high porosity.
Classification
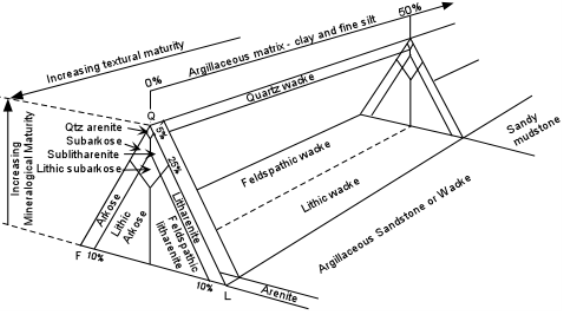
Fig 2.67 Classification of sandstone
Sandstones that contain less than 10% clay matrix are called arenites (note that the Spanish word for sand is arena). These can be subdivided based on the percentages of Quartz, Feldspar, and unstable lithic fragments (fragments of preexisting rock).
A feldspar-rich sandstone is called arkose. Lithic-rich sandstones are called litharenites. Further subdivisions are shown in the diagram. If the rock has between 10 and 50% clay matrix, the rock is called a wacke. Quartz wackes have predominantly quartz surrounded by a mud or clay matrix. In a feldspathic wacke, feldspar is more abundant, and in a lithic wacke, lithic fragments are more abundant. The term graywacke is seldom used today but was originally used to describe a lithic-rich sandstone with between 10 and 50% mica, clay, or chlorite matrix. Rocks with greater than 50% clay matrix are called sandy mudstones and will be discussed in the lecture on mudrocks.
As the percentage of quartz increases, the mineralogical maturity of arenites increases. Also, as the percentage of clay matrix decreases the degree of sorting increases, and thus the textural maturity increases. Textural maturity also increases in the opposite direction as the % clay matrix increases from 50 to 100%.
Mineralogic Composition of Sandstones
As seen in the classification scheme, sandstones are composed of mostly quartz, feldspar, and lithic fragments. Other minerals also occur, depending on the mineralogical maturity of the sandstone. It is these minerals that make studies of the provenance (origin of the grains) possible in the study of sandstones
Greater than 2/3 of the minerals found in sandstones is quartz. There are several reasons for this:
Quartz is one of the most abundant minerals in crystalline rocks like granitoids, schists, and gneisses.
Quartz is mechanically durable due to its high hardness and lack of cleavage.
Quartz is chemically stable under conditions present at the Earth's surface. It has very low solubility in water.
Quartz occurs as both monocrystalline grains and polycrystalline grains and usually shows undulatory extinction.The undulatory extinction is due to deformation either of the preexisting rock from which the grains were derived or results from deformation of the sandstone itself. Thus, even though some workers claim that quartz showing undulatory extinction is derived from a metamorphic source, such quartz cannot be a reliable indicator of a metamorphic source.
Polycrystalline quartz of sand size, especially if more than five individual crystals are present, is a better indicator of a metamorphic source.
Milky quartz is not very common in sandstones, but when it does occur it is likely an indicator that the quartz was derived from a pegmatite or vein quartz. The milky color of such quartz is due to fluid-filled bubbles within the quartz.
Milky quartz, polycrystalline quartz grains, and quartz with undulatory extinction are less stable in the sedimentary environment than monocrystalline non-undulatory quartz. Thus, a sandstone consisting of monocrystalline quartz that does not show undulatory extinction is mineralogically the most mature.
Although feldspars are the most common minerals in igneous and metamorphic rocks, feldspars are less stable than quartz at conditions near the Earth's surface. Thus feldspars make up only 10-15% of all sandstones. Feldspars in sandstones consist of the following:
Plagioclase - usually showing albite twinning. Such plagioclase can be derived from both igneous and metamorphic sources. If the plagioclase also shows zoning, then it is likely from a volcanic source.
Alkali Feldspar - Orthoclase and microcline are derived from both igneous and metamorphic sources. Sanidine is derived from volcanic sources. Microperthite, the intergrowth of K-rich and Na-rich alkali feldspars, is likely derived from a plutonic igneous source.
Because feldspars are unstable in the sedimentary environment, most feldspars in sandstones show the effects of alteration. This is usually evident as growths of microcrystalline clay minerals along cleavage planes and on the surfaces of the feldspars.
Except for fragments of polycrystalline quartz, lithic fragments are generally unstable in the sedimentary environment, yet, if present in a sandstone gives the best clues to provenance. Any type of rock fragment can be found in sandstone, but some kinds are more common due to the following factors:
Since it is possible that any mineral could be found in sand or sandstone depending on the degree of mineralogical maturity, a variety of other minerals are possible. Some of these can be useful in determining the provenance of the sand. The more common minerals in sandstones, quartz, and feldspar, have densities of less than 2,700 kg/m3, but most accessory minerals, except for muscovite, have densities greater than 3,000 kg/m3. Thus the accessory minerals are usually referred to as heavy minerals. This is convenient because if the sandstone can be desegregated, then the heavy minerals can easily be separated from the quartz and feldspar based on density.
The heavy minerals can be divided into three groups, as shown in the table below. Using this list, the provenance of the sand can sometimes be determined to be from an igneous source or a metamorphic source.
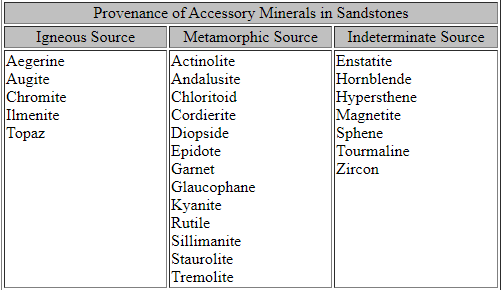
Table 2.9
Glauconite occurs as green or brown sand-sized pellets in some quartz arenites, although sometimes the glauconite pellets make up a substantial portion of the rock. Glauconite has the chemical formula –
(KNaCa)1.2-2.0(Fe+3AlFe+2Mg)4(Si7-7.6Al1-0.4)020(OH)2.nH2O,
although some so-called glauconite sands are composed of such minerals as smectite clays, serpentine, and chlorite. The pellets are thought to originate as fecal pellets. They commonly occur in sands deposited in shallow water (up to 2,000 m) and are most common in Cambro-Ordovician and Cretaceous marine rocks, times when sea level was unusually high and the continents were flooded with epeiric seas. Because glauconite contains K, the sands can sometimes be dated by the K-Ar method of radiometric dating.
Tectonics and Sandstone Compositions
The main factor that creates the basins necessary to form clastic sedimentary rocks is tectonics. Once a basin is formed, the area surrounding the basin will shed its erosional debris and the sediment transported and deposited could form a sandstone. Clues to the tectonic setting in which the basin formed may be left in this accumulated sediment.
Sediments formed from a magmatic arc that has not undergone extensive erosional dissection should consist of a high proportion of volcanic lithic fragments that contain a high ratio of plagioclase to alkali feldspar. With increasing erosional dissection, more plutonic rocks will become exposed and the sediment shed will contain a higher proportion of quartz and alkali feldspar.
Sands derived from sources on continental blocks could come from two tectonic settings. If the continental block has recently split as a result of continental rifting, the sands will be quartzo-feldspathic with high ratios of alkali feldspar to plagioclase. If the sands are derived from high topographic areas located long distances from the depositional areas, the sands will be more quartz-rich, showing a higher degree of mineralogical maturity.
If the source area has recently undergone a major orogenic event, the sands will contain a significant fraction of lithic fragments, with more lithic fragments being derived from parts of the orogenic belt rich in oceanic components and less lithic rich sands from continental sources.
Climate and Sandstones
Climate is controlled largely by latitudinal position on the surface of the Earth and by distance away from the oceans. Humid tropical climates generally occur nearer the equator, and arid to semiarid climates generally occur farther from the oceans and at subtropical latitudes.
Since climate controls the weathering processes, with deeper more intense weathering occurring in humid climates than in arid climates, we might expect to see differences in these conditions showing up in the sediment.
Looking at modern sands derived from plutonic igneous rocks, we find that humid climates produce sands with higher proportions of quartz and lower proportions of lithic fragments than do semiarid climates. Similarly, for sands derived from metamorphic source rocks, humid climates produce more quartz-rich sands than do semiarid climates.
Diagenesis of Sandstones
Once the sand has been deposited and buried by more sediment, it begins to undergo diagenetic processes which can turn the unconsolidated material into sedimentary rock. There are seven main diagenetic processes:
Note that diagenesis is not restricted to sandstones and conglomerates, but occurs in carbonates and mudrocks as well.
Mudstone is a pleasant-grained sedimentary rock consisting of an aggregate of clay and silt-sized particles. Terms including claystone and siltstone are often used in the location of mudstone, although these consult with rocks whose grain length falls inside a good deal narrower stages and underneath near exam those are frequently technically mudstones. Shale is frequently used to explain mudstones that are difficult and fissile (wreck along bedding planes). Marl is regularly used to explain carbonate-rich tender mudstones.
Texture: Clastic Sedimentary Rock
Grain size: Very fine-grained (< 0.06mm); clasts not visible to the naked eye.
Hardness: Generally quite soft, but can be hard and brittle.
Colour – variable – black, white, grey, brown, red, green, blue, etc.
Clasts: Generally a mixture of clay minerals with any or all of quartz, feldspar ( orthoclase,plagioclase), mica ( biotite, chlorite, muscovite); can contain iron oxides (cause red or yellowish coloring); black coloring due to carbonaceous content and/or pyrite.
Other features – smooth to touch.
Mudstone can be separated into these categories:
Clastic sedimentary rocks are classified by the size of the sediment particles making up the rock. Particle size descriptions like sand, silt, and clay have a specific meaning in geology and engineering.
Shales, mudstones, and claystone are rock types that are very similar to each other.
The difference between mudstone and shale is that mudstones break into blocky pieces whereas shales break into thin chips with roughly parallel tops and bottoms.
The terms shale and claystone are sometimes used interchangeably.
Siltstone
As its name implies, it is primarily composed (greater than 2/3) of silt-sized particles, defined as grains 2–62 µm- more than half of the composition is silt-sized particles.

Fig 2.68 Siltstone
Claystone
Claystone is lithified, and non-fissile mudrock. For a rock to be considered claystone, it must consist of up to fifty percent clay, which measures less than 1/256 of a millimeter in particle size — more than half of the composition is clay-sized particles.

Fig 2.69 Claystone
Mudstone
Mudstones and shales are made of silt- and clay-sized particles that are too small to see. The only difference between mudstone and shale is that mudstones break into blocky pieces whereas shales break into thin chips with roughly parallel tops and bottoms. Both are made of ancient mud.
Shale: Exhibits lamination or fissility.
Shale is a fine-grained, clastic sedimentary rock composed of mud that is a mix of flakes of clay minerals and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite. Shale is characterized by breaks along thin laminae or parallel layering or bedding less than one centimeter in thickness, called fissility. It is the most common sedimentary rock
Argillite: It has undergone low-grade metamorphism.
Argillaceous rocks are lithified muds and oozes. They contain variable amounts of silt-sized particles. The argillites grade into shale when the fissile layering typical of shale is developed. Another name for poorly lithified argillites is mudstone. These rocks, although variable in composition, are typically high in aluminium and silica with variable alkali and alkaline earth cations. The term pelitic or pelite is often applied to these sediments and rocks. Metamorphism of argillites produces slate, phyllite, and pelitic schist.
Shale is a fine-grained sedimentary rock that forms from the compaction of silt and clay-size mineral particles that we commonly call "mud." This composition places shale in a category of sedimentary rocks known as "mudstones." Shale is distinguished from other mudstones because it is fissile and laminated. "Laminated" means that the rock is made up of many thin layers. "Fissile" means that the rock readily splits into thin pieces along the laminations.
Uses of Shale
Some shales have special properties that make them important resources. Black shales contain organic material that sometimes breaks down to form natural gas or oil. Other shales can be crushed and mixed with water to produce clays that can be made into a variety of useful objects.

Fig 2.70 Conventional Oil and Natural Gas Reservoir
Conventional Oil and Natural Gas
Black organic shales are the source rock for many of the world's most important oil and natural gas deposits. These shales obtain their black color from tiny particles of organic matter that were deposited with the mud from which the shale formed. As the mud was buried and warmed within the earth, some of the organic material was transformed into oil and natural gas.
The oil and natural gas migrated out of the shale and upwards through the sediment mass because of their low density. The oil and gas were often trapped within the pore spaces of an overlying rock unit such as a sandstone (see illustration). These types of oil and gas deposits are known as "conventional reservoirs" because the fluids can easily flow through the pores of the rock and into the extraction well.
Although drilling can extract large amounts of oil and natural gas from the reservoir rock, much of it remains trapped within the shale. This oil and gas are very difficult to remove because it is trapped within tiny pore spaces or adsorbed onto clay mineral particles that make up the shale.
Shale in brick and tile:
Shale is used as a raw material for making many types of brick, tile, pipe, pottery, and other manufactured products. Brick and tile are some of the most extensively used and highly desired materials for building homes, walls, streets, and commercial structures. Image copyright.
Shale Used to Produce Clay
Everyone has contact with products made from shale. If you live in a brick house, drive on a brick road, live in a house with a tile roof, or keep plants in "terra cotta" pots, you have daily contact with items that were probably made from shale.
Many years ago these same items were made from natural clay. However, heavy use depleted most of the small clay deposits. Needing a new source of raw materials, manufacturers soon discovered that mixing finely ground shale with water would produce clay that often had similar or superior properties. Today, most items that were once produced from natural clay have been replaced by almost identical items made from clay manufactured by mixing finely ground shale with water.
Shale Used to Produce Cement
Cement is another common material that is often made with shale. To make cement, crushed limestone and shale are heated to a temperature that is high enough to evaporate off all water and break down the limestone into calcium oxide and carbon dioxide. The carbon dioxide is lost as an emission, but the calcium oxide combined with the heated shale makes a powder that will harden if mixed with water and allowed to dry. Cement is used to make concrete and many other products for the construction industry.

Fig 2.71 Oil shale
Oil Shale
Oil shale is a rock that contains significant amounts of organic material in the form of kerogen. Up to 1/3 of the rock can be solid kerogen. Liquid and gaseous hydrocarbons can be extracted from oil shale, but the rock must be heated and/or treated with solvents. This is usually much less efficient than drilling rocks that will yield oil or gas directly into a well. Extracting the hydrocarbons from oil shale produces emissions and waste products that cause significant environmental concerns. This is one reason why the world's extensive oil shale deposits have not been aggressively utilized.
Composition of Shale
Shale is a rock composed mainly of clay-sized mineral grains. These tiny grains are usually clay minerals such as illite, kaolinite, and smectite. Shale usually contains other clay-sized mineral particles such as quartz, chert, and feldspar. Other constituents might include organic particles, carbonate minerals, iron oxide minerals, sulfide minerals, and heavy mineral grains. These "other constituents" in the rock are often determined by the shale's environment of deposition, and they often determine the color of the rock.
Colors of Shale
Like most rocks, the color of shale is often determined by the presence of specific materials in minor amounts. Just a few percent of organic materials or iron can significantly alter the color of the rock.
Black and Gray Shale
Black color in sedimentary rocks almost always indicates the presence of organic materials. Just one or two percent organic materials can impart a dark gray or black color to the rock. Besides, this black color almost always implies that the shale is formed from sediment deposited in an oxygen-deficient environment. Any oxygen that entered the environment quickly reacted with the decaying organic debris. If a large amount of oxygen was present, the organic debris would all have decayed. An oxygen-poor environment also provides the proper conditions for the formation of sulfide minerals such as pyrite, another important mineral found in most black shales.
The presence of organic debris in black shales makes them the candidates for oil and gas generation. If the organic material is preserved and properly heated after burial, oil and natural gas might be produced. The Barnett Shale, Marcellus Shale, Haynesville Shale, Fayetteville Shale, and other gas-producing rocks are all dark gray or black shales that yield natural gas. The Bakken Shale of North Dakota and the Eagle Ford Shale of Texas are examples of shales that yield oil.
Gray shales sometimes contain a small amount of organic matter. However, gray shales can also be rocks that contain calcareous materials or simply clay minerals that result in graycolor.
Red, Brown, and Yellow Shale
Shales that are deposited in oxygen-rich environments often contain tiny particles of iron oxide or iron hydroxide minerals such as hematite, goethite, or limonite. Just a few percent of these minerals distributed through the rock can produce the red, brown, or yellow colors exhibited by many types of shale. The presence of hematite can produce a red shale. The presence of limonite or goethite can produce a yellow or brown shale.
Green Shale
Green shales are occasionally found. This should not be surprising because some of the clay minerals and micas that make up much of the volume of these rocks are typically greenish.
Hydraulic Properties of Shale
Hydraulic properties are characteristics of rock such as permeability and porosity that reflect its ability to hold and transmit fluids such as water, oil, or natural gas.
Shale has a very small particle size, so the interstitial spaces are very small. They are so small that oil, natural gas, and water have difficulty moving through the rock. Shale can therefore serve as a cap rock for oil and natural gas traps, and it also is an aquiclude that blocks or limits the flow of groundwater.
Although the interstitial spaces in shale are very small, they can take up a significant volume of the rock. This allows the shale to hold significant amounts of water, gas, or oil but not be able to effectively transmit them because of the low permeability. The oil and gas industry overcomes these limitations of shale by using horizontal drilling and hydraulic fracturing to create artificial porosity and permeability within the rock.
Some of the clay minerals that occur in shale can absorb or adsorb large amounts of water, natural gas, ions, or other substances. This property of shale can enable it to selectively and tenaciously hold or freely release fluids or ions.
Engineering Properties of Shale Soils
Shales and the soils derived from them are some of the most troublesome materials to build upon. They are subject to changes in volume and competence that generally make them unreliable construction substrates.
Landslide: Shale is a landslide-prone rock.
Expansive Soils
The clay minerals in some shale-derived soils can absorb and release large amounts of water. This change in moisture content is usually accompanied by a change in volume which can be as much as several percent. These materials are called "expansive soils." When these soils become wet they swell, and when they dry out they shrink. Buildings, roads, utility lines, or other structures placed upon or within these materials can be weakened or damaged by the forces and motion of volume change. Expansive soils are one of the most common causes of foundation damage to buildings in the United States.
Slope Stability
Shale is the rock most often associated with landslides. Weathering transforms the shale into clay-rich soil which normally has a very low shear strength - especially when wet. When these low-strength materials are wet and on a steep hillside, they can slowly or rapidly move downslope. Overloading or excavation by humans will often trigger failure.
Environments of Shale Deposition
An accumulation of mud begins with the chemical weathering of rocks. This weathering breaks the rocks down into clay minerals and other small particles which often become part of the local soil. A rainstorm might wash tiny particles of soil from the land and into streams, giving the streams a "muddy" appearance. When the stream slows down or enters a standing body of water such as a lake, swamp, or ocean, the mud particles settle to the bottom. If undisturbed and buried, this accumulation of mud might be transformed into a sedimentary rock known as "mudstone." This is how most shales are formed.
The shale-forming process is not confined to Earth. The Mars rovers have found lots of outcrops on Mars with sedimentary rock units that look just like the shales found on Earth
Limestone, sedimentary rock composed mainly of calcium carbonate (CaCO3), usually in the form of calcite or aragonite. It may contain considerable amounts of magnesium carbonate (dolomite) as well; minor constituents also commonly present include clay, iron carbonate, feldspar, pyrite, and quartz.

Fig 2.72 limestone
Most limestones have a granular texture. Their constituent grains range in size from 0.001 mm (0.00004 inches) to visible particles. In many cases, the grains are microscopic fragments of fossil animal shells.

Fig 2.73 sandstone: laminated
Limestone has two origins: (1) biogenic precipitation from seawater, the primary agents being lime-secreting organisms and foraminifera; and (2) mechanical transport and deposition of preexisting limestones, forming clastic deposits. Travertine, tufa, caliche, chalk, sparite, and micrite are all varieties of limestone.
Limestone has long fascinated earth scientists because of its rich fossil content. Much knowledge of the Earth’s chronology and development has been derived from the study of fossils embedded in limestone and other carbonate rocks. Limestone also has considerable commercial importance. Limestones enriched in phosphate by the chemical action of ocean waters constitute a principal source of raw materials for the fertilizer industry. When heated to temperatures of 900 to 1,000 °C (1,650 to 1,800 °F), limestones will dissociate calcium carbonate and yield carbon dioxide and lime, the latter having major applications in the manufacture of glass and agriculture. Certain varieties of limestone also serve as a building stone; they are widely used for flooring, exterior and interior facings, and monuments.
Metamorphism is defined as follows:
The mineralogical and structural adjustment of solid rocks to physical and chemical conditions that have been imposed at depths below the near-surface zones of weathering and diagenesis and which differ from conditions under which the rocks in question originated.
The word "Metamorphism" comes from the Greek: meta = after, morph = form, so metamorphism means the after form. In geology, this refers to the changes in mineral assemblage and texture that result from subjecting a rock to conditions such as pressures, temperatures, and chemical environments different from those under which the rock originally formed.
Note that Diagenesis is also a change in the form that occurs in sedimentary rocks. In geology, however, we restrict diagenetic processes to those which occur at temperatures below 200oC and pressures below about 300 MPa (MPa stands for Mega Pascals), this is equivalent to about 3 kilobars of pressure (1kb = 100 MPa).
Metamorphism, therefore, occurs at temperatures and pressures higher than 200oC and 300 MPa. Rocks can be subjected to these higher temperatures and pressures as they are buried deeper in the Earth. Such burial usually takes place as a result of tectonic processes such as continental collisions or subduction.
The upper limit of metamorphism occurs at the pressure and temperature where the melting of the rock in question begins. Once melting begins, the process changes to an igneous process rather than a metamorphic process.
Grade of Metamorphism
As the temperature and/or pressure increases on a body of rock we say the rock undergoes prograde metamorphism or that the grade of metamorphism increases. The metamorphic grade is a general term for describing the relative temperature and pressure conditions under which metamorphic rocks form.
Low-grade metamorphism takes place at temperatures between about 200 to 320°C, and relatively low pressure. Low-grade metamorphic rocks are generally characterized by an abundance of hydrous minerals. With the increasing grade of metamorphism, the hydrous minerals begin to react with other minerals and/or break down to less hydrous minerals.
High-grade metamorphism takes place at temperatures greater than 320°C and relatively high pressure. As the grade of metamorphism increases, hydrous minerals become less hydrous, by losing H2O, and non-hydrous minerals become more common.
Types of Metamorphism
Contact metamorphism occurs adjacent to igneous intrusions and results from high temperatures associated with the igneous intrusion.
Since only a small area surrounding the intrusion is heated by the magma, metamorphism is restricted to the zone surrounding the intrusion, called a metamorphic or contact aureole. Outside of the contact aureole, the rocks are not affected by the intrusive event. The grade of metamorphism increases in all directions toward the intrusion. Because the temperature contrast between the surrounding rock and the intruded magma is larger at shallow levels in the crust where pressure is low, contact metamorphism is often referred to as high temperature, low-pressure metamorphism. The rock produced is often a fine-grained rock that shows no foliation, called hornfels.
Regional metamorphism occurs over large areas and generally does not show any relationship to igneous bodies. Most regional metamorphism is accompanied by deformation under non-hydrostatic or differential stress conditions. Thus, regional metamorphism usually results in forming metamorphic rocks that are strongly foliated, such as slates, schists, and gneisses. The differential stress usually results from tectonic forces that produce compressional stresses in the rocks, such as when two continental masses collide. Thus, regionally metamorphosed rocks occur in the cores of fold/thrust mountain belts or eroded mountain ranges. Compressive stresses result in the folding of rock and thickening of the crust, which tends to push rocks to deeper levels where they are subjected to higher temperatures and pressures.
Cataclastic metamorphism occurs as a result of mechanical deformation, like when two bodies of rock slide past one another along a fault zone. Heat is generated by the friction of sliding along such a shear zone, and the rocks tend to be mechanically deformed, being crushed and pulverized, due to the shearing. Cataclastic metamorphism is not very common and is restricted to a narrow zone along which the shearing occurred.
Rocks that are altered at high temperatures and moderate pressures by hydrothermal fluids are hydrothermally metamorphosed. This is common in basaltic rocks that generally lack hydrous minerals. The hydrothermal metamorphism results in an alteration to such Mg-Fe rich hydrous minerals as talc, chlorite, serpentine, actinolite, tremolite, zeolites, and clay minerals. Rich ore deposits are often formed as a result of hydrothermal metamorphism.
When sedimentary rocks are buried to depths of several kilometers, temperatures greater than 300°C may develop in the absence of differential stress. New minerals grow, but the rock does not appear to be metamorphosed. The main minerals produced are often the Zeolites. Burial metamorphism overlaps, to some extent, with diagenesis, and grades into regional metamorphism as temperature and pressure increase.
When an extraterrestrial body, such as a meteorite or comet impacts the Earth or if there is a very large volcanic explosion, ultrahigh pressures can be generated in the impacted rock. These ultrahigh pressures can produce minerals that are only stable at very high pressure, such as the SiO2 polymorphs coesite and stishovite. Besides, they can produce textures known as shock lamellae in mineral grains, and such textures as shatter cones in the impacted rock.
Classification of Metamorphic Rocks
Classification of metamorphic rocks is based on mineral assemblage, texture, protolith, and bulk chemical composition of the rock. Each of these will be discussed in turn, then we will summarize how metamorphic rocks are classified.
In metamorphic rocks, individual minerals may or may not be bounded by crystal faces. Those that are bounded by their crystal faces are termed idioblastic. Those that show none of their crystal faces are termed xenoblastic. From the examination of metamorphic rocks, it has been found that metamorphic minerals can be listed in a generalized sequence, known as the crystalloblastic series, listing minerals in order of their tendency to be idioblastic. In the series, each mineral tends to develop idioblastic surfaces against any mineral that occurs lower in the series. This series is listed below:
This series can, in a rather general way, enable us to determine the origin of a given rock. For example, a rock that shows euhedral plagioclase crystals in contact with anhedral amphibole, likely had an igneous protolith, since a metamorphic rock with the same minerals would be expected to show euhedral amphibole in contact with anhedral plagioclase.
Another aspect of the crystalloblastic series is that minerals high on the list tend to form porphyroblasts (the metamorphic equivalent of phenocrysts), although K-feldspar (a mineral that occurs lower in the list) may also form porphyroblasts. Porphyroblasts are often riddled with inclusions of other minerals that were enveloped during the growth of the porphyroblast. These are said to have a poikioblastic texture.
Most metamorphic textures involve foliation. Foliation is generally caused by a preferred orientation of sheet silicates. If a rock has a slatey cleavage as its foliation, it is termed a slate, if it has a phyletic foliation, it is termed a phyllite, if it has a shistose foliation, it is termed schist. A rock that shows a banded texture without a distinct foliation is termed a gneiss. All of these could be porphyroblastic (i.e. could contain porphyroblasts).
A rock that shows no foliation is called a hornfels if the grain size is small, and a granulite if the grain size is large and individual minerals can be easily distinguished with a hand lens.
Protolith
Protolith refers to the original rock, before metamorphism. In low-grade metamorphic rocks, original textures are often preserved allowing one to determine the likely protolith. As the grade of metamorphism increases, original textures are replaced with metamorphic textures and other clues, such as the bulk chemical composition of the rock, is used to determine the protolith.
Bulk Chemical Composition
The mineral assemblage that develops in a metamorphic rock is dependent on
Just like in igneous rocks, minerals can only form if the necessary chemical constituents are present in the rock (i.e. the concept of silica saturation and alumina saturation applies to metamorphic rocks as well). Based on the mineral assemblage present in the rock one can often estimate the approximate bulk chemical composition of the rock. Some terms that describe this general bulk chemical composition are as follows:
These rocks are derivatives of aluminous sedimentary rocks like shales and mudrocks. Because of their high concentrations of alumina, they are recognized by an abundance of aluminous minerals, like clay minerals, micas, kyanite, sillimanite, andalusite, and garnet.
Rocks that originally contained mostly quartz and feldspars like granitic rocks and arkosic sandstones will also contain an abundance of quartz and feldspar as metamorphic rocks since these minerals are stable over a wide range of temperature and pressure. Those that exhibit mostly quartz and feldspar with only minor amounts of aluminous minerals are termed quartzo-feldspathic.
Calcareous rocks are calcium-rich.They are usually derivatives of carbonate rocks, although they contain other minerals that result from the reaction of the carbonates with associated siliceous detrital minerals that were present in the rock. At low grades of metamorphism calcareous rocks are recognized by their abundance of carbonate minerals like calcite and dolomite. With the increasing grade of metamorphism, these are replaced by minerals like brucite, phlogopite (Mg-rich biotite), chlorite, and tremolite. At even higher grades anhydrous minerals like diopside, forsterite, wollastonite, grossularite, and calcic plagioclase.
Just like in igneous rocks, the general term basic refers to low silica content. Basic metamorphic rocks are generally derivatives of basic igneous rocks like basalts and gabbros. They have an abundance of Fe-Mg minerals like biotite, chlorite, and hornblende, as well as calcic minerals like plagioclase and epidote.
Rocks that are rich in Mg with relatively less Fe, are termed magnesian. Such rocks would contain Mg-rich minerals like serpentine, brucite, talc, dolomite, and tremolite. In general, such rocks usually have an ultrabasic protolith, like peridotite, dunite, or pyroxenite.
Rocks that are rich in Fe with little Mg are termed ferruginous. Such rocks could be derivatives of Fe-rich cherts or ironstones. They are characterized by an abundance of Fe-rich minerals like greenalite (Fe-rich serpentine), minnesotaite (Fe-rich talc), ferroactinolite, ferrocummingtonite, hematite, and magnetite at low grades, and ferrosilite, fayalite, ferrohedenbergite, and almandine garnet at higher grades.
Rocks that are characterized by the presence of Mn-rich minerals are termed manganiferous. They are characterized by such minerals as Stilpnomelane and spessartine.
Classification
Classification of metamorphic rocks depends on what is visible in the rock and its degree of metamorphism. Note that classification is generally loose and practical such that names can be adapted to describe the rock in the most satisfactory way that conveys the important characteristics. Three kinds of criteria are normally employed. These are:
In addition to these conventions, certain non-foliated rocks with specific chemical compositions and/or mineral assemblages are given specific names. These are as follows:
Certain rocks can get split into thin sheets along closely spaced parallel planes which may be inclined to the bedding planes. Such a tendency of rock is called rock cleavage. Rock cleavage is seen in foliated rocks formed by the action of direct pressure and generally accompanied by some recrystallization and elongation with the constituent minerals in a parallel arrangement.
Metamorphic rocks like schists and slate have this characteristic property of rock cleavage. However, this property is also exhibited by shales and limestone’s under shear stresses and low temperature.
The degree of cleavage depends on the degree of the parallel arrangement of the constituents and the inequality of size of the constituent grains. Cleavages are observed normal to the direction of greater pressure or in the plane of action of shear stresses which may be at some inclination to the direction of greatest pressure.
Types of Rock Cleavage:Two types of rock cleavages can exist in the rocks, namely, flow cleavage and fracture cleavage.
1. Flow Cleavage:
This cleavage shows not only partings between the oriented mineral grains but also partings of cleavage planes of the minerals. This type of cleavage is characteristic of slate and some varieties of schists exhibiting the property in the whole mass.
This type of cleavage is also called Schistosity or Slaty cleavage. Flow cleavage happens in conditions of large strain formations under no change in volume and is a typical plastic deformation with recrystallization.
While in the somewhat plastic state the rock gets elongated parallel to the cleavages accompanied by contractions at right angles. The orientations of the cleavages are normal to the direction of greatest stress. Fracture cleavage is caused by shearing in highly strained rocks as in rocks buried at great depth.
2. Slaty Cleavage:
This is a type of cleavage characteristic of slate and some rocks rich in micaceous minerals. In this case, the rock readily splits with smooth flat surfaces. Slate mainly consists of finely divided flaky minerals like mica, chlorite, and tabular minerals like feldspar and quartz. Under the action of pressure, these minerals are flattened and consequently elongated in the process of metamorphic transformations.
Slaty cleavage occurs in shale when subjected to pressure so that it is first folded and subsequently the mineral grains are elongated and turned. The cleavage planes are oriented parallel to the axial planes of the folds.
Fig 2.74 Slaty Cleavage
Importance of Rock Cleavage:The following points cover the importance of rock cleavage:
(i) Rock cleavage provides an additional structural plane of weakness in addition to the bedding planes and joint planes.
(ii) Rock cleavages allow the rocks to be split along closely spaced parallel planes. This helps easy quarrying and obtaining thin slabs. Ex: Slate, chlorite, schist.
(iii) Slates and such cleaved rocks, some types of limestone’s which are bedded and jointed if present on hillsides render a dangerously un-stale state.
(iv) Slates and schists with cleavages undergo surface creep.
The layering in a coarse-grained, crystalline rock due to the parallel arrangement of platy mineral grains such as muscovite and biotite. Other minerals present are typically quartz and feldspar, plus a variety of other minerals such as garnet, staurolite,kyanite, sillimanite.
At intermediate and high grades of metamorphism, the chlorite breaks down and recrystallizes to form quartz, feldspar, and mica. The grain size also increases and individual mineral grains can be seen with the unaided eye.
Foliation in coarse-grained metamorphic rocks is called schistosity. In a hand sample, the foliation can be easily seen, and usually runs planar through the rock; that is, it all runs the same direction. In larger specimens, however, the foliation may be folded. Schistosity is derived from the Latin schistose meaning cleaves easily. Schistosity differs from slaty cleavage in both grain size and mineral content.
Gneiss, a metamorphic rock that has a distinct banding, which is apparent in hand specimens or on a microscopic scale. Gneiss usually is distinguished from a schist by its foliation and schistosity; gneiss displays a well-developed foliation and a poorly developed schistosity and cleavage. For the casual student, it is convenient to think of gneiss as a rock with parallel, somewhat irregular banding which has little tendency to split along planes. In contrast, schist typically is composed of platy minerals with a parallel to a subparallel geometric orientation that gives the rock a tendency to split along planes; banding is usually not present.

Fig 2.75 Gneiss
Gneiss is a type of metamorphic rock with distinct banding due to the presence of differing proportions of minerals in the various bands.
Gneiss is medium- to coarse-grained and may contain abundant quartz and feldspar, which some petrographers regard as essential components. The banding is usually due to the presence of differing proportions of minerals in the various bands; dark and light bands may alternate because of the separation of mafic (dark) and felsic (light) minerals. Banding can also be caused by differing grain sizes of the same minerals. The mineralogy of a particular gneiss is a result of the complex interaction of original rock composition, pressure and temperature of metamorphism, and the addition or loss of components.
Gneiss is the principal rock over extensive metamorphic terrains. The banding may be oriented nearly parallel to the Earth’s surface (horizontal dip) or may have a steep dip. Such orientations can be interpreted in terms of the stresses that prevailed during the formation of the rock.

Fig 2.76 Banded gneiss.
Gneiss can be classified based on minerals that are present, presumed formational processes, chemical composition, or probable parent material. Orthogneiss is formed by the metamorphism of igneous rocks; paragneiss results from the metamorphism of sedimentary rocks. Pencil gneiss contains rod-shaped individual minerals or segregations of minerals, and Augen gneiss contains stubby lenses of feldspar and quartz having the appearance of eyes scattered through the rock. The identification of gneiss as a product of metamorphism is usually clear, but some primary gneiss can be formed by the flow of a viscous, partially crystallized magma.
Schist is a medium-grade metamorphic rock, formed by the metamorphosis of mudstone/shale, or some types of igneous rock, to a higher degree than slate, i.e. it has been subjected to higher temperatures and pressures. The resulting foliation is coarser and more distinct than that of slate due to the higher degree of crystallization of mica minerals ( biotite, chlorite, muscovite) forming larger crystals, and is often referred to as schistosity. These larger crystals reflect light so that schist often has a high luster, i.e. it is shiny. Porphyroblasts are common in schist, and they provide information on the temperature and pressure conditions under which the rock is formed. Due to the more extreme formation conditions, schist often shows complex folding patterns. There are many varieties of schist and they are named for the dominant mineral comprising the rock, e.g. mica schist, greenschist (green because of high chlorite content), garnet schist, etc.

Fig 2.79 Schist
Texture - foliated, foliation on mm to cm scale.
Grain size - fine to medium-grained; can often see crystals with the naked eye.
Hardness - generally hard.
Colour - variable - often alternating lighter and darker bands, often shiny.
Mineralogy - mica minerals ( biotite, chlorite, muscovite), quartz, and plagioclase often present as monomineralic bands, garnet porphyroblasts common.
Other features - generally smoothish to touch.
Uses - generally used as decorative rock, e.g. walls, gardens, etc; a high percentage of mica group minerals precludes its use in the construction and roading industries.
Slate is a fine-grained, foliated metamorphic rock that is created by the alteration of shale or mudstone by low-grade regional metamorphism. It is popular for a wide variety of uses such as roofing, flooring, and flagging because of its durability and attractive appearance.
Composition of Slate
Slate is composed mainly of clay minerals or micas, depending upon the degree of metamorphism to which it has been subjected. The original clay minerals in shale alter to micas with increasing levels of heat and pressure. Slate can also contain abundant quartz and small amounts of feldspar, calcite, pyrite, hematite, and other minerals.

Fig 2.80 Slate roof
Color of Slate
Most slates are gray in color and range in a continuum of shades from light to dark gray. Slate also occurs in shades of green, red, black, purple, and brown. The color of the slate is often determined by the amount and type of iron and organic material that are present in the rock.
Formation
The tectonic environment for producing slate is usually a former sedimentary basin that becomes involved in a convergent plate boundary. Shales and mudstones in that basin are compressed by horizontal forces with minor heating. These forces and heat modify the clay minerals in the shale and mudstone. Foliation develops at right angles to the compressive forces of the convergent plate boundary to yield a vertical foliation that usually crosses the bedding planes that existed in the shale.

Fig 2.81 School slate
Uses of the Word "Slate"
The word "slate" has not been used consistently over time and in some industries. Today most geologists are careful not to use the word "slate" when talking about "shale." However, in the past, the word slate was often used freely about shale.
This confusion of terms partially arises from the fact that shale is progressively converted into slate. Imagine driving your car eastwards in Pennsylvania through areas of increasing metamorphism, starting where the rock is definitely "shale" and stopping to examine rock at each outcrop. You will have a difficult time deciding where on that route "shale" has been converted into "slate." It can be difficult to pick up a rock and apply the proper name where the rocks have been lightly metamorphosed.
In the coal mining industry of the Appalachian Basin, the word "slate" is still used by many miners about the shale that forms the roof and floor of a mine, and for fragments of shale that are separated from the coal in preparation plants. Experienced miners train newer miners, and archaic language is passed along.
In the 1800s, elementary school students used a small piece of slate mounted in a wooden frame for writing practice and arithmetic problems. The writing was done with a small pencil made of slate, soapstone, or clay. The slate could be wiped clean with a soft cloth. Small slates were also used in schools and businesses to list daily events, schedules, menus, prices, and other notices. Today, over 150 years after writing slates started to disappear from schools, the word "slate" is still used in phrases such as "clean slate," "wipe the slate clean," "slated for today," "put it on the slate" and more.
Slaty Cleavage
Foliation in slate is caused by the parallel orientation of platy minerals in the rock, such as microscopic grains of clay minerals and mica. These parallel mineral grain alignments give the rock the ability to break smoothly along planes of foliation. People exploit this property of slate to produce thin sheets of slate that are used in construction projects and manufacturing.

Fig 2.82 Slate tile flooring.
Uses of Slate
Most of the slate mined throughout the world is used to produce roofing slates. Slate performs well in this application because it can be cut into thin sheets, absorbs minimal moisture, and stands up well in contact with freezing water. A disadvantage is a cost of the slate and its installation in comparison with other roofing materials. As a result, in new construction slate is mainly confined to high-end projects and prestigious architecture.
Slate is also used for interior flooring, exterior paving, dimension stone, and decorative aggregate. Small pieces of slate are also used to make turkey calls. The photos on this page document several uses of slate. Historically slate has been used for chalkboards, student writing slates, billiard tables, cemetery markers, whetstones, and tabletops. Because it is a good electrical insulator, it was also used for early electric panels and switch boxes.
References: