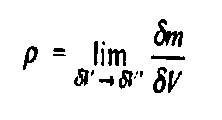Unit - 1
Basic concept
From the macroscopic viewpoint, we are always concerned with volume which is very large compared to molecular dimensions. Even a very small volume of a system is assume to contain a large number of molecule so that statically averaging is meaningful and a property value can be assign to it. Disregarding the behaviour of individual molecules, matter is here treated as continuous.
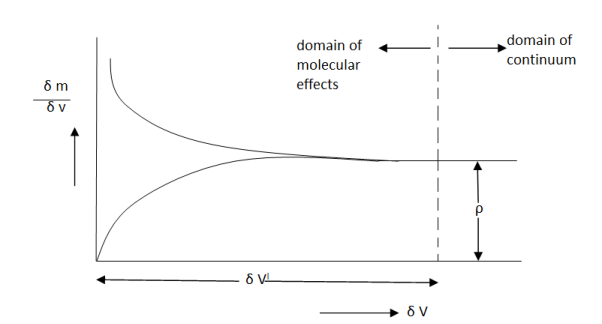
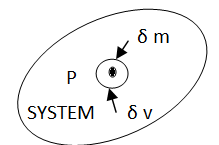
Let us consider a mass ∂m in a volume ∂V surrounded the point P (Fig 1). The ratio ∂m/∂V is the average mass density of the system within the volume ∂V. we suppose that at a first ∂V is rather large and is subsequently shrunk about the point P. If we plot ∂m/∂V against ∂V becomes so small as to contain relatively few molecules, the average density fluctuates substantially with time as molecules pass into and out of the volume in random motion, and so it is impossible to speak of a definite value of ∂m/∂V. The smallest volume which may be regarded as continuous is ∂V’. The density ρ of the system at a point is thus defined as
| |
|
|
Fig 1 Definition of the macroscopic property, density
Similarly, the fluid velocity at a point P is defined as the instantaneous velocity of the centre of gravity of the smallest continuous volume ∂V’.
The concept of continuum loses validity when the mean free path of the molecules approaches the order of magnitude of the dimensions of the vessel, as, for instance, in highly rarefied gases encountered in high vacuum technology, in rocket flights at high altitudes and in electron tubes. In most engineering applications however the assumption of a continuum is valid and convenient, and goes hand in hand with macroscopic point of view.
There are two points of view from which the behavior of matter can be studied: the macroscopic and the microscopic. In the macroscopic approach a certain quantity of matters is considered, without the events occurring at the molecular level being taken into account. From the microscopic point of view matter is composed of myriads of molecules. If it is a gas, each molecule at a given instant has a certain position, velocity, and energy, and for each molecule these changes very frequently as a result of collisions. The behaviour of gas is described by summing up the behaviour of each molecule. Such a study is made in microscopic or statistical thermodynamics. Macroscopic thermodynamics is only concerned with the effect of the action of many molecules, and these effects can be perceived by human senses. For example the macroscopic quantity, pressure, is the average rate of change of momentum due to all the molecular collision made on a unit area. The effect of pressure can be felt. The macroscopic point of view is not concerned with the action of individual molecule, and the force on a given unit area can be measured by using e.g. a pressure gauge. These macroscopic observations are completely independent of assumptions regarding the nature of matter. All the results of classical or macroscopic thermodynamics can, however, be derived from the microscopic and statistical study of matter.
Thermodynamic systems
System
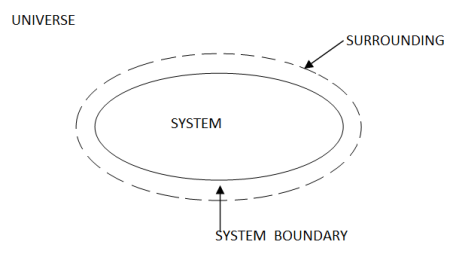
Whenever a change is to be analysed it is essential to specify the region in which the change take place. This is done by drawing a boundary aroundthe region. Boundary may be real or imaginary.
Everything within the boundary is called the system. The region outside the boundary is called surroundings
|
Fig 2
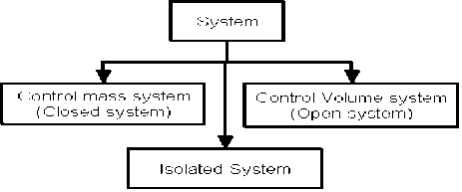
There are three types of Thermodynamics systems:
|
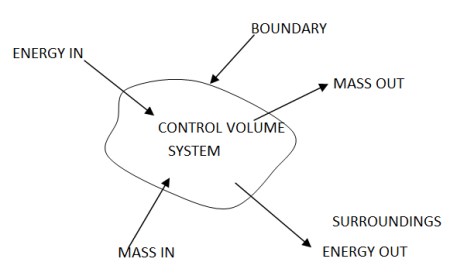
1 – Open system (control volume system): If mass and energy both cross the boundary of a system it is called open system .in open system both mass and energy can transfer across the boundaries and the mass within the system may not be constant .most of the engineering devices are open systems involving flow of fluids through them.
|
Fig 3
Example of open system
- Boiler – In boiler water convert from liquid to vapour phase. So mass and energy both transfer the boundary.
- Hydraulic turbine wheel – In this device potential energy of water convert into mechanical works. Water enters the wheel from head race side and leaves it to the tail race from the other end, and as such mass cross the system boundary.
- Motor-car engine – The engine sucks charge which is mixture of air and petrol and finally exhausts the gases to the surrounding atmosphere.
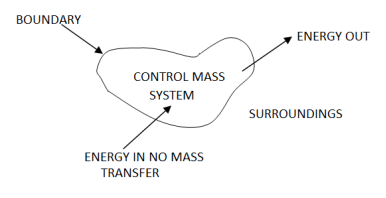
2 – Closed system (control mass system): If the mass within the boundary of the system does not change, it is called closed system. A closed system does not allow any mass transfer across the boundary but allow transfer of energy across the boundary.
|
Fig 4
Example of closed system
- Piston cylinder assembly –the gas can reject or receive heat, expand or contract but no mass cross the boundary of system.
- Ignition system – electric energy cross the boundary to cause a spark between electrodes an initiate combustion .there is heat transfer but not mass across the system.
Pressure cooker, refrigerator, ice cream freezer etc. are the example of closed system.
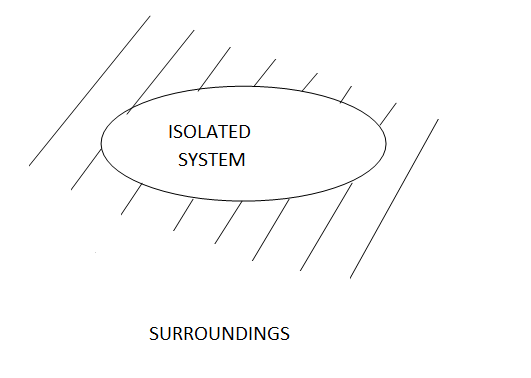
3 - Isolated system: Neither mass nor energy cross the boundary of a system .it is called an isolated system. In this no mass and energy cross the system
|
Fig 5
Example of isolated system
- Universe can be considered as an isolated system
- Fluid enclosed in a perfectly insulated closed vessel (thermos flask)
Thermodynamic Properties– Intensive and Extensive
Intensive property: If the property is independent of mass of the system is called an intensive property.
Example - Pressure, temperature, density, velocity, height, viscosity are the example of intensive property.
Extensive property: If the property is proportional to the mass of the system it is called an extensive property.
Example-volume, surface area, potential energy, kinetic energy, internal energy, electric charge
State:
The application of thermodynamic principles begins by defining a system that is in some sense distinct from its surroundings. For example, the system could be a sample of gas inside a cylinder with a movable piston, an entire steam engine, a marathon runner, the planet Earth, a neutron star, a black hole, or even the entire universe. In general, systems are free to exchange heat, work, and other forms of energy with their surroundings.
A system’s condition at any given time is called its thermodynamic state. For a gas in a cylinder with a movable piston, the state of the system is identified by the temperature, pressure, and volume of the gas. These properties are characteristic parameters that have definite values at each state and are independent of the way in which the system arrived at that state. In other words, any change in value of a property depends only on the initial and final states of the system, not on the path followed by the system from one state to another. Such properties are called state functions. In contrast, the work done as the piston moves and the gas expands and the heat the gas absorbs from its surroundings depends on the detailed way in which the expansion occurs.
The behaviour of a complex thermodynamic system, such as Earth’s atmosphere, can be understood by first applying the principles of states and properties to its component part in this case, water, water vapour, and the various gases making up the atmosphere. By isolating samples of material whose states and properties can be controlled and manipulated, properties and their interrelations can be studied as the system changes from state to state.
Path: If all the change of states of system are plotted and all the points are conned, then the line joining the change of states of the system is called the path
Process:
Thermodynamics process represents a transition in which a system changes from one state to another. When the path is completely specified then the change of state is called a process. A Process is defined as the transformation of the system from one fixed state to another fixed state .When any one of the properties changes, the working substance or system is said to have undergone a process.
Some of the processes are identified by special names as given below:
- Isobaric process (constant pressure process)
- Isochoric process (constant volume process)
- Isothermal process (constant temperature process)
- Isentropic process (constant entropy process)
- Adiabatic process(perfectly insulated process)
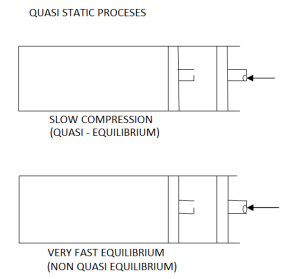
If process is carried out Infinity slowly then it is called as Quasistatic process.
When a process proceeds in such a manner that the system remains infinitesimally close to an equilibrium state at all times: Quasi-static or Quasi-equilibrium process The process proceeds slow enough to allow the system to the system to adjust itself internally so that properties in one part of the system do not change any faster than those at other parts.
It is a thermodynamic process which occurs infinitesimal slowly. Every state passed by the quasi static process tends to equilibrium. Quasi means almost and static means the thermodynamic properties static with time. Quasi static process is also a reversible process.
|
|
|
Fig 6
Infinite slowness is characteristic feature of quasi static process.
If the gradient is present in system properties then the process cannot be quasi static for a finite time .but if the time for the process is infinite then the process can be a quasi-static although there is some gradient
|
Fig 7
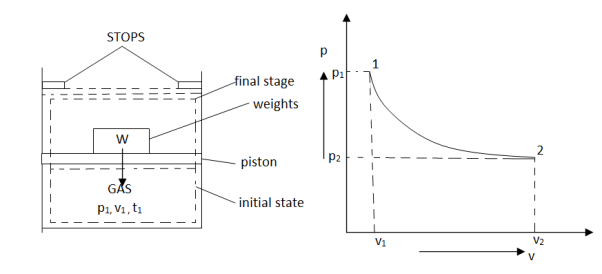
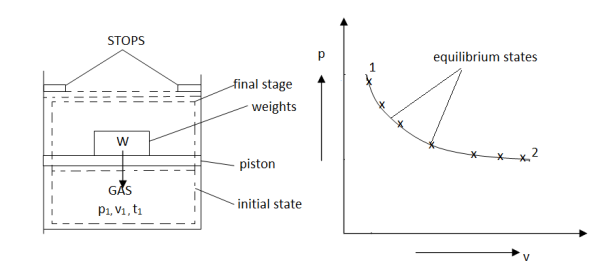
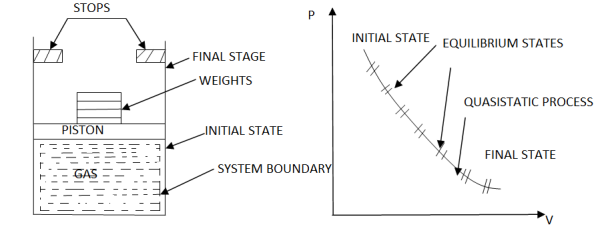
As in figure the piston cylinder assembly is shown and the gas is in equilibrium by the constant pressure (weight) applied on piston and it's state can be represented by thermodynamic properties p1, v1, t1.
If the weights are suddenly removed then the gas will force the piston upwards until the stops or another equilibrium state.
The final equilibrium state can be represented by thermodynamic properties p2,v2,t2.
Now the inbetween states are not in equilibrium as the spontaneous process occurs.If the weights are now divided into infinite parts and it is removed one by one so that the process moves on through the successive equilibrium states.
This is the example of quasi static process.
Work:In thermodynamics, work performed by a system is the energy transferred by the system to its surroundings. Kinetic energy, potential energy and internal energy are forms of energy that are properties of a system. Work is a form of energy, but it is energy in transit. A system contains no work, work is a process done by or on a system. In general, work is defined for mechanical systems as the action of a force on an object through a distance.
Work done by a system on its surrounding is considered positive while work done on the system by the surroundings is considered negative
We know that
Work = force x displacement
But in case of thermodynamics works we take force is the product of a pressure p at the boundary and the area A of the moving boundary therefore
dW = pAdl
Where dW is the work done during an infinite displacement dl of the boundary
Since Adl = dv = change in volume
dW = pdv
The conditions under which the above expression is valid are
- The system is closed
- There are no viscous effects within the system.
- The pressure and other properties have the same value on all boundaries of the system
- The effects due to gravity electricity or magnetism are negligible
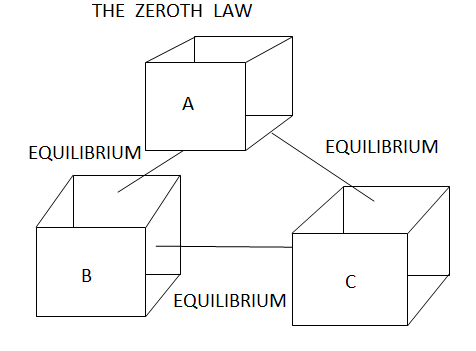
If two systems are each in thermal equilibrium with the third system then the two systems are in thermal equilibrium which each other.
|
|
Figure 8 The Zeroth law of thermodynamics
When a body A is in thermal equilibrium with a body B , and also separately with a body C ,then B and C will thermal equilibrium with each other .this is known as the Zeroth law of thermodynamics.
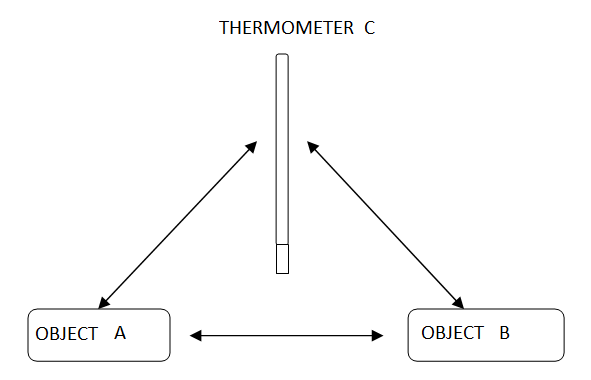
We use the Zeroth law when we wish to compare the temperatures of two objects, A and B. We can do this by using a thermometer, C and placing it again object A it reaches thermal equilibrium with object A and measure the temperature of A. Placing the thermometer against object B until thermal equilibrium is reached we measure the temperature of object B. If they are the same temperature then they will be in thermal equilibrium with each other.
Heat
Heat is the transfer of kinetic energy from one medium or object to another or from an energy source to a medium or object. Such energy transfer can occur in three ways: radiation, conduction, and convection.
The standard unit of heat in the International System of Units (SI) is the calorie (cal), which is the amount of energy transfer required to raise the temperature of one gram of pure liquid water by one degree Celsius, provided the water temperature is higher than the freezing point and lower than the boiling point. Sometimes the kilocalorie (kcal) is specified as a unit of heat; 1 kcal = 1000 cal. (This is the so-called diet calorie.) Less often, the British thermal unit (Btu) is used. This is the amount of heat required to raise the temperature of one pound of pure liquid water by one degree Fahrenheit.
An example of heat by radiation is the effect of infrared (IR) energy as it strikes a surface. IR is an electromagnetic field capable of transferring energy from a source, such as a fireplace, to a destination, such as the surfaces within a room. Radiation does not require an intervening medium; it can occur through a vacuum. It is responsible for the warming of the Earth by the sun.
Heat by conduction takes place when two material media or objects are in direct contact, and the temperature of one is higher than the temperature of the other. The temperatures tend to equalize; thus the heat conduction consists of a transfer of kinetic energy from the warmer medium to the cooler one. An example is the immersion of a chilled human body in a hot bath.
Heat by convection occurs when the motion of a liquid or gas carries energy from a warmer region to a cooler region. A good example of convection is the tendency of warm air to rise and cool air to fall, equalizing the air temperature inside a room containing a hot stove. Heat convection (along with conduction) is believed to take place inside the Earth, transferring kinetic energy from the inner core through the outer core and mantle to the crust. In this situation, the outer core and the mantle behave like liquids over long periods of time.
Temperature
Temperature is the degree or intensity of the heat present in a substance or a system, expressed based on the comparative scale and shown by a thermometer.In other words, Temperature is the measure of hotness or coldness of a body measured using Celsius, Kelvin, and Fahrenheit scales.
The change in temperature is based on the amount of heat released or absorbed. The S.I unit of temperature is Kelvin.
The Temperature formula is given by,
Δ T = Q / mc
Where,
Δ T = temperature difference,
Q = amount of heat absorbed or released,
m = mass of the body,
c = specific heat of the body.
IDEAL GAS
An ideal gas is a theoretical gas composed of a set of randomly-moving point particles that interact only through elastic collisions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.
At normal ambient conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Generally, deviation from an ideal gas tends to decrease with higher temperature and lower density, as the work performed by intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them.
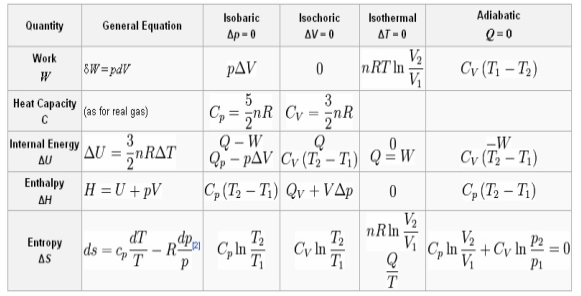
EQUATION TABLE FOR AN IDEAL GAS
|
Fig 9
REAL GAS
Real gas, as opposed to a Perfect or Ideal Gas, effects refers to an assumption base where the following are taken into account:
•Compressibility effects
•Variable heat capacity
•Van der Waals forces
•Non-equilibrium thermodynamic effects
•Issues with molecular dissociation and elementary reactions with variable composition.
For most applications, such a detailed analysis is "over-kill" and the ideal gas approximation is used. Real-gas models have to be used near condensation point of gases, near critical point, at very high pressures, and in several other less usual cases.
Reference:
- Engineering thermodynamics
- ASHRAE, Air Conditioning System Design Manual, IInd edition, ASHRAE.
- Thermodynamics P.K Nag
- Threlkeld J.L., Thermal Environmental Engineering, Prentice Hall Inc. New Delhi