Module II
Air
The invisible gaseous substance that surrounds the earth is called Air. It is an essential part of life for all living beings. However, this most essential life supporting element can get polluted. Presence of one or more contaminants in the ambient atmosphere can not only have an adverse effect on human beings, plants & animals but also on non-living objects.
The presence of one or more contaminants, such as dust, fumes, gas, mist, odour, smoke or vapour, in the air in large quantities and for sufficient duration such that it is injurious to human, plant or animal life or to property and can also interfere with comfortable enjoyment of life & property, is termed as Air Pollution.
Composition of Air
When non-polluted, the composition of air should be, as follows:
Gases | Percentage Amount in Air |
Nitrogen | 78.09 |
Oxygen | 20.95 |
Argon | 0.93 |
CO2 | 0.03 |
Other Gases | Traces |
Air also contains a variable amount of water vapour, on average around 1% at sea level, and 0.4% over the entire atmosphere.
Properties of Air
Air is a mixture of gases, water vapor, and other substances, and it has specific properties:
- Air is made up of gases. The composition of these gases has been discussed above.
- Air has mass and occupies space.
- Air exerts pressure and has weight.
- Air can be compressed.
- Air is affected by temperature & altitude.
Sources of Air Pollutants
1) On the Basis of Origin of Source
a) Natural Contaminants: dust storms, forest fires, ocean sprays, pollen grains etc.
b) Anthropogenic or Man-made Contaminants: Industries, automobiles etc.
2) On the Basis of Spatial Distribution of Source
a) Point Source: Chimneys, thermal power station etc.
b) Areal Source: Industrial area, Residential area etc.
3) On the Basis of Position of Source
a) Stationary Source: Factories, power plants etc.
b) Mobile Source: Automobiles
Classification of Air Pollutants
1) On the Basis of Origin
a) Primary Air Pollutants: These are emitted directly from identifiable sources. Examples are oxides of sulphur (SO2); oxides of carbon (CO, CO2); oxides of nitrogen (NO, NO2); Lead (Pb); Hydrocarbons, H2S, H2F, Ethyl & Methyl Mercaptan, Halogen compounds, Organic Compounds, Radioactive Compounds
b) Secondary Air Pollutants: These are produced due to interaction between two or more primary pollutants or by reaction with normal atmospheric constituents, with or without photo-activation. Examples are H2SO4, Ozone (O3), Formaldehydes, PAN (Per-oxy acetyl nitrate)
2) On the Basis of Nature of Pollutants
a) Organic Pollutants: Hydrocarbons
b) Inorganic Pollutants: SOx, NOx, CO etc
3) On the Basis of State of Matter
a) Particulates: solid particulates (dust, smoke, fumes etc.); liquid particulates (mist, spray); aerosols etc.
b) Gases: SOx, NOx, CO etc
Properties of Major Air Pollutants
- Particulates: Dispersed microscopic solid or liquid particles (0.01 µm to 200 µm) in gaseous media such as dust, smoke, mist etc. It can be better understood as a colloidal system in which the dispersion medium is a gas and the dispersed phase is liquid or solid. Particulates in atmosphere are measured by High Volume Air Sampler, using the following equation:
Total Suspended Particle (µg/m3) = 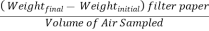
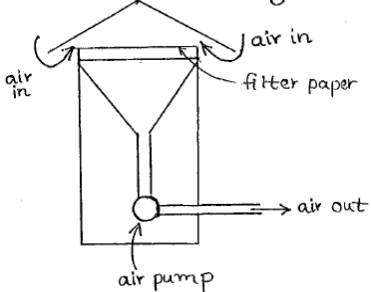
Figure 1: High Volume Air Sampler
Aerosols are solid & liquid particulates of size ≤ 1 µm dispersed in the atmosphere. The term ‘aerosol’ applies only till the pollutant remains dispersed in air. It no longer remains an air pollutant after it settles. Thus particulate matter is an air pollutant only when it is an aerosol. The various types of aerosols are, as follows:
DUST
- Made up of solid particles ranging from 1 to 1000 µm.
- Produced by crushing & grinding of organic & inorganic materials.
- Mostly, the dust particles settle onto the ground due to their large sizes but particles ≤ 5 µm remain suspended in air.
SMOKE
- Made up of finely divided particles ranging from 0.5 to 1 µm.
- Produced by incomplete combustion and is hence, predominantly made up of carbon particles.
- Mostly, the dust particles settle onto the ground due to their large sizes but particles ≤ 5 µm remain suspended in air.
MIST
- Made up of water droplets of size 50 to 100 µm in diameter suspended in the atmosphere.
- It is a low concentration dispersion of water droplets of large size.
FOG
- If mist concentration is high enough to obscure visibility, it is termed as fog.
- Made up of water or ice droplets of size ≤ 50 µm in diameter dispersed in the atmosphere, near the Earth’s surface.
SMOG (Smoke + Fog)
- It is of 3 types: Photochemical Smog, Coal induced Smog, Modern/ Traffic Smog.
- Photochemical Smog: caused due to reaction between hydrocarbons & oxides of nitrogen under the influence of sunlight giving rise to toxic peroxy acetyl nitrate (PAN). It reduces visibility, causes eye irritation, damages vegetables and causes cracking of rubber.
- Coal induced Smog: consists of smoke, fly ash and sulphur compounds.
- Modern/ Traffic Smog: produced from vehicular emissions and industrial fumes that react in the atmosphere in the presence of sunlight, to form secondary pollutants.
FUMES
- Extremely fine solid particles of size 0.03 to 0.3 µm.
- Produced from condensation of vapours due to volatilisation of molten substances.
2. Gases:
OXIDES OF SULPHUR
- Most important air pollutant.
- SO2 and SO3 are most dangerous and can damage buildings, plants and act as respiratory irritant.
- Produced by combustion of fuels, especially coal in refineries, chemical plants, incineration plants etc.
- Presence measured by West & Gaeke Method.
HYDROGEN SULPHIDE & MERCAPTANS
- H2S is a foul smelling gas, emitted during volcanic eruptions, anaerobic decay of living matter and in pulp industry.
- Mercaptans are also sulphur compounds emitted from petroleum refineries, pulp mills and chemical plants. Since mercaptans have a strong odour, it can be mixed in gases to detect leakages.
HYDROGEN FLUORIDE
- Produced by manufacturing of phosphate fertilisers, aluminium industry, brick plants etc.
- Can cause damage to vegetation, animals & human beings.
OXIDES OF NITROGEN
- There are 7 oxides of Nitrogen (N2O, NO, NO2, NO3, N2O3, N2O4, N2O5) but only NO and NO2 are termed as pollutants.
- Emitted as effluent from industries that produce nitric acid and from automobile exhaust.
- Presence is measured by the Jacob - Oscheisser method.
CARBON MONOXIDE
- An odourless and colourless gas produced due to incomplete combustion of carbonaceous materials.
- Highly poisonous and termed as an asphyxiant (suffocating effect) when it reacts with haemoglobin in blood.
- Emitted from automobile exhaust.
- Presence is analysed by NDIR analyser (Non-dispersive Infrared).
HYDROCARBONS & ALDEHYDES
- Produced by combustion of gasoline, diesel, fuel oil and natural gas and can also be formed in the atmosphere because of photochemical reactions.
- Presence is analysed by Gas Chromatography.
Monitoring of Air Pollutants is an exercise to measure ambient air pollution levels in an area. It does not reduce air pollution but indicates how much is the pollution, where is the pollution, and when is that pollution. Concentration of air pollutants is expressed in terms of ppm (parts per million) or ppb (parts per billion) or µg/m3 (micro-gram per cubic metre).
The Air Quality Index (AQI) helps to indicate the status of the quality of air we breathe.
AQI Range | Levels of Health Concern |
0 to 50 | Good |
51 to 100 | Moderate |
101 to 150 | Unhealthy for Sensitive Groups |
151 to 200 | Unhealthy |
201 to 300 | Very Unhealthy |
301 to 500 | Hazardous |
The data obtained over a long period of time, includes spatial differences in pollution (which areas of the city are more polluted or better) and temporal differences (is there a pattern of pollution levels during the day and/or over the seasons).
Central Pollution Control Board (estd. 1974) has executed a nation-wide programme of ambient air quality monitoring known as National Air Quality Monitoring Programme (NAMP) in 1984-85. Under NAMP, four air pollutants viz., Sulphur Dioxide (SO2), Oxides of Nitrogen (NO2), Respirable Suspended Particulate Matter (RSPM/PM10) and Fine Particulate Matter (PM2.5) and have been identified for regular monitoring at all the locations. Currently, the network consists of 793 operating stations to carry out the following tasks:
- Determine status and trends of ambient air quality
- Ascertain whether the prescribed ambient air quality standards are violated
- Determine the non-attainment cities
- Obtain the knowledge and understanding necessary for developing preventive and corrective measures
- Monitor meteorological parameters such as wind speed and wind direction, relative humidity (RH) and temperature
Type of Air Pollution Monitoring | Focus Area |
Ambient | Whole city, state or country |
On-Road | Roads & their vicinity |
Satellite | Whole city, state or country |
Emissions | Specific Source |
There are certain diseases which are related to one’s occupation. These are caused by constant use of certain substances that sneak into air and then enter our body. An occupational disease is a chronic ailment that occurs as a result of an occupational activity. It is an aspect of occupational safety and health. The various occupational hazards related to industrial air pollution are:
- Silicosis (Silico-tuberculosis) occurs due to inhalation of free silica, or SiO2 (Silicon dioxide). It is prevalent in mining industries and those related to pottery, ceramic, glass, building and construction work. The workers tend to suffer from chronic cough and chest pain. Silicosis may not be curable but it is 100% preventable, if exposure is minimized.
- Asbestosis is caused by inhaling asbestos, which is used in making ceilings. It is a cancer causing agent. The disease is characterized by progressive and irreversible respiratory disability. It may also lead to death due to cardiac failure.
- Byssinosis or the brown lung disease is an occupational respiratory disorder due to inhalation of cotton fibres over a long period of time. It is characterized by the narrowing of pulmonary airways. It is a disabling lung disease, which is marked by chronic cough and chronic bronchitis.
- Coal worker’s Pneumoconiosis or the black lung disease occurs due to inhalation of coal dust from coal mining industry.
- In industries manufacturing batteries, cable sheaths, shipbuilding, lead oxide, radiation protection etc., workers tend to inhale lead fumes which over time may gastrointestinal damage, liver and kidney damage, abnormalities in fertility etc.
- Nitrogen Oxide produced during burning of coal, oil and diesel fuel may lead to eye and nasal irritation and pulmonary discomfort.
Preventive Measures:
The most effective way to prevent occupational respiratory diseases is to minimize exposure. However, this is not a practical approach from the perspective of industries where industrial dust is an unavoidable by-product. In such cases, certain stringent safety protocols need to be implemented to effectively curtail exposure to the hazardous dust sources.
The Indian Council of Medical Research-National Institute of Occupational Health (ICMR-NIOH) has been working for many years towards improving the management of occupational health risks in India. ICMR-NIOH is an occupational health research institute funded by the Government of India that is responsible for helping the national policy makers to develop the most suitable and effective policies for eliminating and reducing cases of serious work-related ill health and disease.
A few recommended precautionary measures to reduce exposure to a variety of industrial dust are as follows:
- Recognize when industrial dust may be generated and plan ahead to eliminate or control the dust at the source. Awareness and planning are keys to prevention of silicosis.
- Do not use silica sand or other substances containing more than 1% crystalline silica as abrasive blasting materials. Substitute less hazardous materials.
- Use engineering controls and containment methods such as blast-cleaning machines and cabinets, wet drilling, or wet sawing of silica-containing materials to control the hazard and protect adjacent workers from exposure.
- Routinely maintain dust control systems to keep them in good working order.
- Practice good personal hygiene to avoid unnecessary exposure to other worksite contaminants such as lead.
- Wear disposable or washable protective clothes at the worksite.
- Shower and change into clean clothes before leaving the worksite to prevent contamination of cars, homes, and other work areas.
- Conduct air monitoring to measure worker exposures and ensure that controls are providing adequate protection for workers.
- Use adequate respiratory protection when source controls cannot keep silica exposures below the designated limit.
- Provide periodic medical examinations for all workers who may be exposed to the different hazardous industrial dust.
- Post warning signs to mark the boundaries of work areas contaminated with the various possible pollutants.
- Provide workers with training that includes information about health effects, work practices, and protective equipment.
Urban areas are susceptible to the accumulation of air pollutants because of the large quantity and diversity of emissions in a concentrated area.
Studies show that traffic (25%), combustion & heating (22%), construction activities (20%), natural dust and salt (18%), and industrial activities (15%) are the main sources of particulate matter contributing to urban air pollution. Urban air quality is highly dependent on meteorological conditions such as temperature, wind, pressure and humidity. These factors affect the dispersion and dilution of pollutants in the atmosphere.
1) Wind
Air in motion is called wind. Horizontal movement & dispersion of air pollutants in atmosphere depends on wind speed and direction. Presence of winds in the atmosphere represents unstable condition. Wind data at a place is represented by the Wind Rose Diagram.
An Anemometer is used to measure wind speed and wind vane gives us the wind direction. Wind speed at any altitude can be found using the 1/7th Law:
Wind (Height)1/7
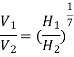
2) Pressure
Cyclones and anticyclones are both wind systems caused due to pressure differences.
A cyclone is an area of low pressure which typically indicates bad weather, like rain and clouds. Winds in a cyclone blow counter-clockwise in the Northern Hemisphere and clockwise in the Southern Hemisphere. Air near the ground is pushed toward the low-pressure centre of the cyclone, and then rises upward, expanding and cooling as it moves. As it cools, the rising air becomes more humid, leading to cloudiness and high humidity within the cyclone.
An anticyclone is an area of high pressure indicating fair weather. Winds in an anticyclone blow clockwise in the Northern Hemisphere and counter-clockwise in the Southern Hemisphere. Air at the centre of an anticyclone is forced away from its area of high pressure and replaced by a downward blast of air from higher altitudes. The air compresses and heats up as it moves downwards thereby reducing its humidity.
3) Humidity
This represents the moisture in the air. Secondary pollutants are formed in the atmosphere only under humid conditions.
4) Temperature & Lapse Rate
In the troposphere, the temperature of the ambient air decreases with increase in height. This rate of change of temperature with altitude is known as Lapse Rate.
Lapse Rate = - 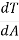
Where, dT = change in temperature
DA = change in altitude
Negative sign indicates that temperature decreases with increase in altitude.
These are of two types:
a) Environmental Lapse Rate (ELR): Rate of change of temperature of ambient air with altitude is known as ELR. It is the rate at which the temperature of ambient air decreases with increase in altitude.
ELR = 6.5ᵒC/ km
b) Adiabatic Lapse Rate (ALR): Temperature of a pollutant also decreases with increase in height above the Earth’s surface. The rate at which it decreases with increase in height is called ALR.
For dry air, dry ALR = 9.8ᵒC/ km
For wet/ saturated air, wet ALR = 6.0ᵒC/ km
Dispersion and dilution of pollutants in the atmosphere depends on the difference between ALR and ELR.
CASE 1: Super Adiabatic Lapse Rate (ELR > ALR)
The environment is said to be in ‘unstable condition’, which is favourable for pollutant dispersion and dilution.
The pollutant has higher energy than the ambient air and hence higher dispersion of the pollutant occurs in random directions, making the environment unstable.
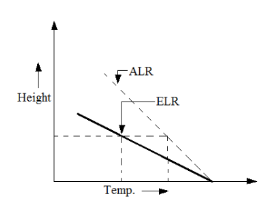
Figure 2: Super Adiabatic Lapse Rate
CASE 2: Sub Adiabatic Lapse Rate (ALR > ELR)
The environment is said to be in ‘stable condition’, which is bad for pollutant dispersion and dilution.
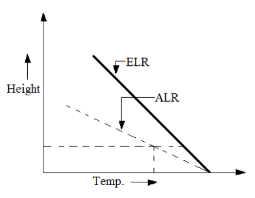
Figure 3: Sub Adiabatic Lapse Rate
CASE 3: Neutral Lapse Rate (ALR = ELR)
The environment is said to be in ‘neutrally stable condition’. This is an ideal condition.
Negative Lapse Rate
Usually around midnight, the temperature of the ambient air increases with increase in height, thus indicating a negative lapse rate, commonly known as Inversion. In this state, warmer air lies above colder air. Such phenomenon occurs for a very short period of time. Inversion is of two types:
a) Radiation Inversion: It occurs when the earth cools down more rapidly than the air above it. This is a nocturnal phenomenon and usually occurs up to a height of few hundred meters. It helps in the formation of fog when air is wet and tends to trap gases and particulate matter. Chimneys are to be provided above the radiation inversion level.
b) Subsidence Inversion: It occurs due to sinking of air in a high pressure area, surrounded by a low pressure area. It usually occurs up to a height of 1500 meters or more.
Plume Behaviour
A plume is the path taken by the continuous discharge of gaseous effluents emitted from a stack or chimney.
a) Looping Plume
- Occurs under super adiabatic lapse rate conditions with moderate wind speed on a hot summer afternoon.
- Plume has wavy behaviour because of highly unstable atmosphere.
- Characterised by high dispersion but causes nuisance as particulates touch the ground.
b) Neutral Plume
- Plume tends to move vertically upwards until it reaches a height of same surrounding temperature & pressure.
- Occurs when ALR = ELR.
c) Coning Plume
- Occurs under sub-adiabatic lapse rate conditions, during cloudy day or night with strong wind velocity.
- Plume shape is vertically symmetrical about the plume line and it reaches the ground at a large distance.
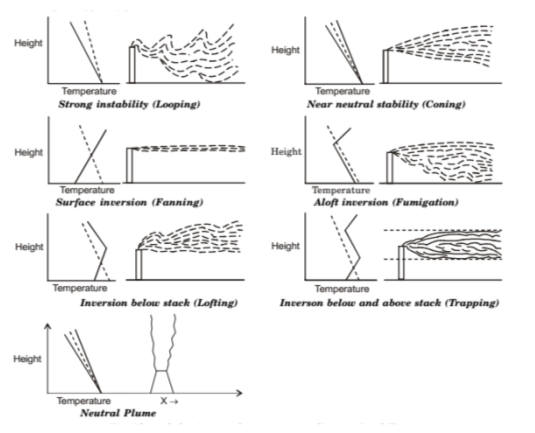
Figure 4: Plume Behaviour under various Stability Conditions
d) Fanning Plume
- Occurs under extreme inversion conditions in the presence of light wind.
- No vertical mixing; only horizontal movement.
e) Lofting Plume
- It is the best condition that occurs when there exists a strong super-adiabatic lapse rate over surface inversion.
- Such plume has minimum downward mixing but the upward mixing will be quite turbulent and rapid.
f) Fumigating Plume
- It is the worst condition that occurs when there exists a strong super-adiabatic lapse rate below surface inversion.
- Such plume has only downward mixing and the pollutants cannot escape the lower atmosphere.
g) Trapping Plume
- Plume is caught between two inversion layers and will be confined between these layers, allowing no mixing in the atmosphere.
- It is considered to be a bad condition for pollutant dispersion.
Automobiles are a necessary evil. On one hand, they have made living easy and convenient while on the other, they have also made human life more complicated and vulnerable to both toxic emissions and an increased risk of accidents.
It has been proven that prolonged exposure to traffic conditions, especially for traffic policemen, may lead to respiratory, digestive, lung and skin disorders.
Sources of Automobile Pollutants:
- Hydrocarbons
- Carbon Monoxide
- Oxides of Nitrogen
- Particulate Matter
- Oxides of Sulphur
Chemistry of Combustion in Automobiles:
For an automobile to move on the road, it must utilise the energy stored in fuel and convert it to mechanical energy to drive the wheels.
Fuel or Gasoline is made up of carbon and hydrogen atoms. During combustion, the carbon from the gasoline reacts with the oxygen in the air to produce carbon dioxide and a large amount of heat. The reaction that occurs during combustion of fuel in automobiles is as follows:
 Fuel (CxHy) + Oxygen (O2) + Spark Water (H2O) + Carbon dioxide (CO2) + Heat
Fuel (CxHy) + Oxygen (O2) + Spark Water (H2O) + Carbon dioxide (CO2) + Heat
This heat that is generated is converted into mechanical energy that propels the vehicle.
Measures to Control Automobile Pollution:
- Reduce use of individual cars and promote car pools and use of public transport.
- Monitor and repair any engine leaks.
- Always take used oil, batteries and other fluids to a repair shop for proper disposal.
- Never allow oil or other toxins to runoff into the ground, street gutters or storm drains.
- When purchasing a new automobile, look for cars with high fuel efficiency ratings.
Quality of Different Types of Fuels used in Automobiles:
1) Gasoline or Petrol
Gasoline is made from petroleum. It is the most common automobile fuel that is used all over the world to power cars, motorcycles, scooters, boats, lawnmowers, and other machinery. It produces high level of CO2 on combustion and is hence considered an outdated fuel now.
2) Diesel
It is also made from petroleum but is refined using a different method than that used to create gasoline. Diesel powered cars typically get better gas mileage or fuel efficiency than gasoline powered vehicles. Also, some drivers feel that they get a better value for their money even if diesel is more expensive.
3) Compressed Natural Gas
Compressed Natural Gas (CNG) (methane stored at high pressure) is a fuel that can be used in place of gasoline, diesel fuel and liquefied petroleum gas (LPG). CNG combustion produces fewer undesirable gases than the aforementioned fuels. In comparison to other fuels, natural gas poses less of a threat in the event of a spill, because it is lighter than air and disperses quickly when released.
4) Ethanol
Although ethanol is not widely used as a general automobile fuel, it is added to gasoline because it is a cost effective fuel. It is made from renewable resources like corn and sugarcane.
5) Bio-diesel
Diesel fuel that is created using vegetable oils or animal fats is called bio-diesel. It can be made using soybean oil, lard, algae, and vegetable oils.
Operating Conditions of Good Quality Fuels used in Automobiles:
- A good fuel should possess high calorific value. Calorific value of the fuel is also called as the heat value of the fuel. It is defined as the amount of heat released by burning of unit quantity of the fuel. In SI system, calorific value is measured as J/kg (Joule per kg). The calorific value of the fuel can be measured by a devise called as Bomb Calorimeter.
- It should have proper ignition temperature. The ignition temperature of the fuel should neither be too low nor too high. The lowest temperature at which a substance catches fire and undergoes combustion liberating heat and light is called ignition temperature.
- It should not produce poisonous products during combustion. In other words, it should not cause pollution on combustion.
- It should have moderate rate of combustion.
- Combustion should be easily controllable i.e., combustion of fuel should be easy to start or stop as and when required.
- It should not leave behind much ash on combustion.
- It should be easily available in plenty.
- It should have low moisture content.
- It should be cheap.
- It should be easy to handle and transport.
Interrelationships between Different Types of Automobile Fuels:
Fuel | Approximate Cost | Calorific value (KJ/kg) | Air Pollutant |
Petrol | Rs. 80.99/litre | 45000 | Yes |
Diesel | Rs. 73.56/litre | 45000 | Yes |
CNG | Rs 50.00/kg | 50000 | No |
Ethanol | Rs 45.40/litre | 25000-30000 | Partially |
Bio-diesel | Rs 40.00/litre | 35000-40000 | No |
Government of India has laid down National Ambient Air Quality Standards (NAAQS) for twelve air pollutants: PM10, PM2.5, Carbon Monoxide (CO), Sulphur Dioxide (SO2), Nitrogen Dioxide (NO2), Ammonia (NH3), Ground Level Ozone (O3), Lead, Arsenic, Nickel, Benzene and Benzo(a)Pyrene.
The NAAQS helps in assessing the quality of air with respect to the above mentioned pollutants. We can thereby develop preventive and corrective measures for mitigation. Compliance to the notified environment standards is likely to protect and improve the quality of the environment.
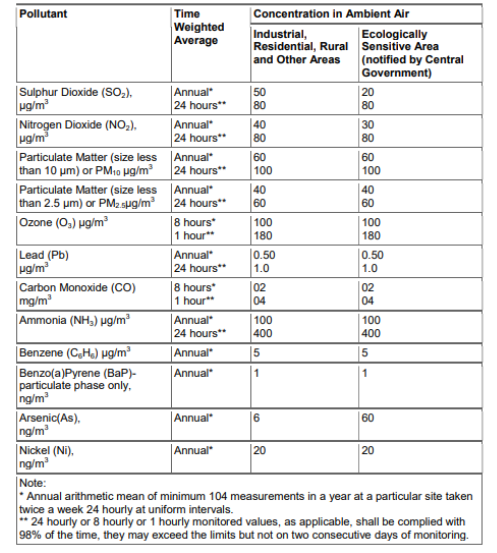
Figure 5: National Ambient Air Quality Standards
a) Acid Rain
Causes
It is produced when sulphur oxides and nitrogen oxides present in the atmosphere interact with water vapour and sunlight and are chemically converted to strong acidic compounds (H2SO4 and HNO3). These acidic compounds, along with some other organic & inorganic compounds, are deposited on the earth as aerosols or in the form of raindrops, snowflakes, fog & dew. If the pH of rainwater is less than 5.6, it is termed as acidic rain.
Effects
Acid rain adversely affects vegetation. It may cause skin diseases in humans and also causes rusting of iron in buildings etc. It also causes leaching of nutrients in soil.
b) Global Warming & Greenhouse Effect
Causes
Global warming occurs when carbon dioxide (CO2) and other air pollutants present in the atmosphere, absorb solar radiations that have bounced off the earth’s surface. Normally, this radiation would escape into space—but these pollutants trap the heat and cause the planet to get hotter. That's what's known as the greenhouse effect. The primary greenhouse gases in Earth's atmosphere are water vapour (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and ozone (O3).
Greenhouse effect has been used to grow plants, such as tomatoes and tropical flowers. A greenhouse is a building with glass walls and a glass roof. It traps sunlight which helps in the growth of plants.
Effects
Temperature of the Earth may increase and polar ice caps may melt vigorously. Flora and fauna may also be affected by the high temperatures.
c) Ozone Layer Depletion
Causes
The ozone layer acts as a protective shield for the earth’s atmosphere, against the harmful UV rays of the Sun. Primary reason for the ozone layer depletion is the Chlorofluorocarbons (CFCs) used as aerosol propellants, refrigerants, solvents etc.
Effects
Ozone layer depletion may cause burns & skin cancer in humans.
- Natural Process
- Dispersion: to reduce the concentration of pollutants in one place
- Settling: to remove particles of size > 20 µm
- Washout or Scavenging: natural absorption process below cloud level in which particulates are collected in rain or mist and settle down with the moisture
- Rainout: occurs within the cloud where water droplets are formed around the particulates
- Adsorption: takes place near the surface of the earth where solid, liquid and gaseous particulates are attracted to the surface and are retained there
2. Engineered Process
- Dilution: tall chimneys discharge the plumes at a higher level from the ground where they get diluted in to the atmosphere
- Control at Source: includes controlling pollution at the source itself with the help of certain pollution control devices
Control Devices for Particulates: Construction & Limitations
- Gravitational Settling Chambers
- It is a simple particulate collection device that works on the principle of gravity to settle the particulate matter in a gas stream passing through its long chamber.
- The primary requirement of this control device is a chamber in which the carrier gas velocity is reduced so as to allow the particulate matter to settle out of the moving gas stream under the action of gravity.
- This particulate matter is then collected at the bottom of the chamber. The chamber is then cleaned manually to dispose the waste.
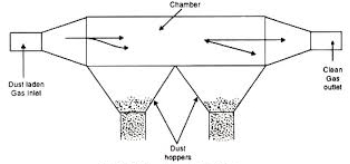
Figure 6: Gravitational Settling Chambers
- Baffle rods and wire mesh screens may be suspended in the chamber to minimize turbulence and to ensure uniform flow.
- Gravitational Settling Chambers are said to remove particulates down to 5-10 µm theoretically and 50 µm practically.
- Settling Velocity of particles (Vs) in the chamber is given by Stoke’s Law:
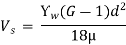
Where, Ƴw = density of gas
G = specific gravity of gas
µ = kinematic viscosity of gas
d = diameter of particle
Limitations of Gravitational Settling Chambers
- Large space requirements
- Only comparatively large particles (greater than 10 micron) can be collected
2. Centrifugal Gas Collectors
- These make use of centrifugal force to separate particulate matter from the gas stream.
- Particles of size smaller than those removed in Gravitational Settling Chamber can be removed here.
- These are of 2 types:
a) Cyclone Collectors
Design: These consist of a cylindrical shell with a conical base, a dust hopper and an inlet for the entry of dust-laden gas.
Working Principle: It works on the principle that the inlet velocity of the gas is transferred into spinning vortex, which helps to throw out the particles under the generated centrifugal force. The greater the magnitude of the force, the higher is the removal efficiency.
Use: These are used in grain mills, cement plants, fertiliser plants, petroleum refineries etc.
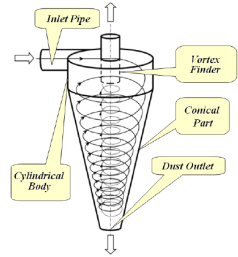
Figure 7: Cyclone Collectors
b) Dynamic Precipitators
Working Principle: It imparts a centrifugal force to the particles in the incoming gas by the rotation of its vanes. It is 7 times more effective than the cyclone collectors.
Use: These are used in ceramics, food & pharmaceutical and wood working industries.
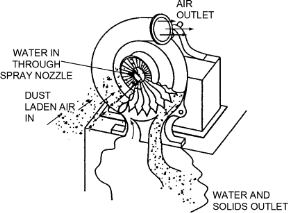
Figure 8: Dynamic Precipitators
Limitations of Centrifugal Gas Collectors
- Low collection efficiency for particles below 5 - 10 µ in diameter
- Severe abrasion problems can occur during the striking of particles on the walls of the cyclone
- Decrease in efficiency at low particulate concentration
3. Wet Scrubbers
- These remove the particulates from the incoming gas stream by allowing flue gases to flow up against a falling water stream.
- The particulates mix with the water droplets and fall down to get removed.
- Wet scrubbers are of 3 types:
a) Spray Towers
Low-cost scrubbers used to remove both gaseous & particulate matter
Can handle large volumes of gases
Cause very little pressure loss
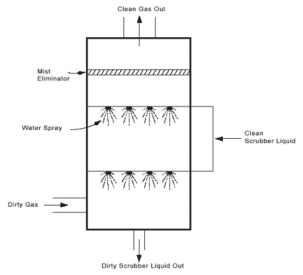
Figure 9: Spray Towers
b) Wet Cyclone Scrubbers
High pressure spray nozzles are placed in a cyclone chamber and eject a fine spray that intercepts the small particles entrained in the swirling gases
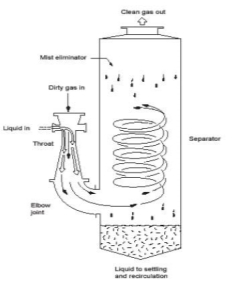
Figure 10: Wet Cyclone Scrubbers
Particulate matter thrown to the wall is then drained to the collection sump
Efficiency higher than that of spray towers
c) Venturi Scrubbers
Contaminated gas passes through a duct with a venture-shaped throat section, at velocity of 60-180 m/s.
Coarse water spray is injected in the throat section where it is atomized by high gas velocities
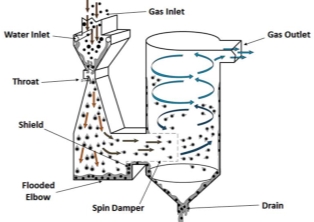
Figure 11: Venturi Scrubbers
Liquid droplets collide with the particulate matter which then falls down for later removal
Most efficient system for removal of particles in the range of 0.5-5 µm.
Limitations of Wet Scrubbers
- High power consumption for higher efficiency
- Moderate to high maintenance costs owing to corrosion and abrasion
- Wet disposal of the collected material
4. Electrostatic Precipitators
- In these, flue gas is passed through a highly ionised atmosphere where the particles get highly charged and then can be removed with the help of electrostatic forces.
- Used in thermal power plants, paper industries, mining industries, iron & steel plants, chemical industries etc.
- Efficiency: 95-99%
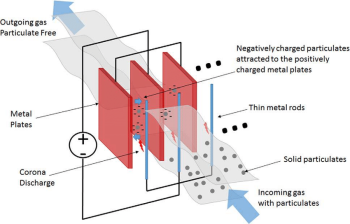
Figure 12: Electrostatic Precipitators
Limitations of Electrostatic Precipitators
- High initial cost
- Space requirement is more because of the large size of the equipment
- Possible explosion hazards during collection of combustible gases or particulate
- Precautions are necessary to maintain safety during operation
- The negatively charged electrodes during gas ionization produce the ozone.
5. Fabric Filters
- Gas stream is passes through a woven fabric which filters out the particulate matter but the gaseous matter flows on
- Used in many high volume operations such as cement kilns, foundries, steel furnaces & grain handling plants
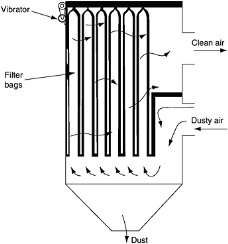
Figure 13: Fabric Filters
Limitations of Fabric Filters
- Operating limits are imposed by high carrier gas temperatures, high humidity and other parameters
- High maintenance and fabric replacement costs
- Bag houses are difficult to maintain because of the difficulty in finding and replacing even a single leaking bag
- Large size of equipment
- Problems in handling dusts which may abrade, corrode, or blind the cloth
Control Devices for Gases: Construction & Limitations
- Adsorption Units
- Pollution-laden gas is made to pass through beds of a porous material
- Pollutants are effectively caught and held onto the surface of the material by physical and chemical adsorption
- Commonly used adsorbents: activated carbon, aluminium, magnesium, silica gel & fullers earth
- It is of 3 types:
a) Fixed Bed Adsorbers:
Container for this kind of adsorber can be horizontal or vertical cylindrical shells
Activated carbon is arranged on beds in 1.3 cm thick layers
b) Moving Bed Adsorbers:
Activated carbon is contained in a rotating drum
Vapour-laden air enters through ports above the carbon bed, passes through the cylindrical carbon bed, enters the space inside of the drum and then leaves through the exit ports
c) Fluidized Bed Adsorbers:
Contains shallow floating bed of adsorbent
Expansion and fluidization of the adsorbent allows intimate contact between the adsorbent and the contaminated gas
Limitations of Adsorption Units
- Adsorbent progressively deteriorates in capacity as number of cycles increases
- Adsorbent regeneration requires a steam or vacuum source
- Relatively high capital cost
- Spent adsorbent may be considered a hazardous waste
- Some contaminants may undergo violent exothermal reactions with adsorbent (explosion danger)
2. Absorption Units
- Pollution-laden gas is brought in contact with a solvent and contaminants in the gas are removed/ treated depending upon the type of contaminant gas and the solvent used
- Example: Aqueous solutions of alkalis and alkaline earths (Na, NH3, MgO, CaO) can help in the removal of SO2
- It is of 4 types: Spray Towers, Plate or Tray Towers, Packed Towers and Venturi Scrubbers
Limitations of Absorption Units
- Spray Towers are less effective in removal of high concentration of gaseous contaminants
- Packed towers may become clogged when high concentration of particulate loads are introduced
3. Condensation Units
- Working Principle: A compound will condense at a given temperature if its partial pressure is increased till it is equal to or greater than its vapour pressure
- Generally considered as a pre-treatment device for air pollution control and is used in conjunction with adsorption and absorption units.
- It is of 2 types: Surface Condenser and Contact Condenser
Limitations of Condensation Units
- An air cooler or cooling tower must be present to provide cooling water
- Variable output is obtained at variable gas composition and fluid level
4. Combustion Units
- This process is used to purify contaminated gases when the admixed pollutants are oxidisable to inert gases
- Hydrocarbons and CO can be easily burnt, oxidised and removed
- It is of 3 types: Direct Flame Combustion, Thermal Combustion and Catalytic Combustion
Reference Books:
1) Santosh Kumar Garg -“Environmental Engineering (Vol. II) Sewage Waste Disposal and Air Pollution Engineering” – Khanna Publishers
2) Dr. K.N. Duggal- “Elements of Environmental Engineering” – S. Chand
Reference YouTube Video Links:
- Https://www.youtube.com/watch?v=jwjc7Pw_KC4
- Https://www.youtube.com/watch?v=4AuwG2G_ERU&list=PLF5457B8AE71516CE