UNIT 4
Biomolecules
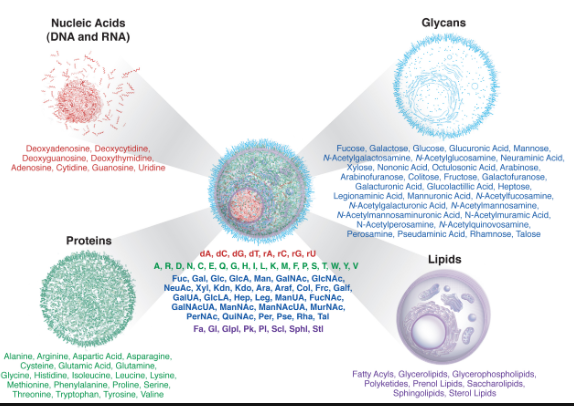
Fig: 1 The four molecules of life are proteins, lipids, carbohydrates and nucleic acids. Each of the four groups is vital for every single organism present on Earth. Without any of these four molecules, a cell and organism would not exist.
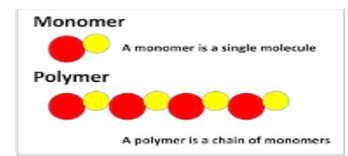
5. A polymer is derived from (Greek poly "many" + mer, "part") is a large molecule, or macromolecule, composed of several repeated subunits. In daily life the synthetic and natural polymers play a very important role that may be specific in nature due to their broad range of properties.
6. The synthetic and natural polymers are formed by polymerisation of many smaller molecules known as monomers, the Polymers range from natural biopolymers such as DNA and proteins that are fundamental to biological structure and function and genetic transfer to synthetic plastics such as polystyrene. The polymers have a large molecular mass, and relatively are small molecule compounds, therefore they produce unique physical properties that are used for various purposes including toughness, viscoelasticity, and a specific tendency to form glasses and semi crystalline structures rather than crystals.
7. Polymers are studied widely in the fields of macromolecular science and biophysics, and polymer science. Historically, when observed the products that arise from the linkage of repeating units bound by covalent chemical bonds have been the primary focus of polymer science; this is emerging as the important areas of science that now focuses on non-covalent links.
8. Polyisoprene obtained from latex rubber is a fine example of a biological polymers, and a good example of synthetic polymer is polystyrene of Styrofoam. In biological contexts, essentially all biological macromolecules—i.e., proteins (polyamides), nucleic acids (polynucleotides), and polysaccharides—are purely polymeric in nature

Fig: 2 Sugar is a sweet crystallizable material that consists essentially or wholly of sucrose, is colourless or white when it is in pure and tends to be brown when less refined, is obtained commercially from sugarcane or sugar beet
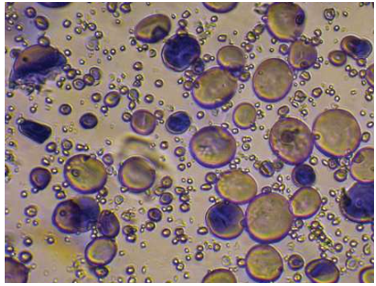
Fig 3: Starch granules present in wheat grain; the starch granules are stained with iodine.
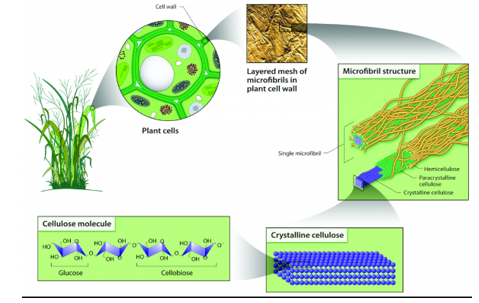
Fig 4: shows the cellulose molecule structure, they are the basic structural units of all plant cells
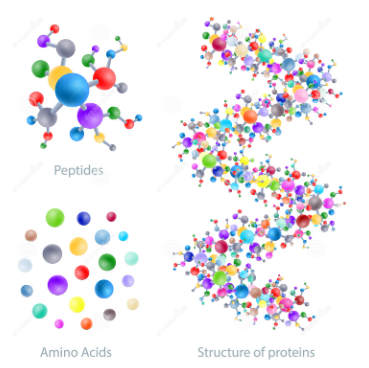
Fig 5 : The pictures show Amino Acids and the compounds are bound together by peptide bonds, all proteins are made up of one or more chains of amino acids.
3. The common protein is important in the biology of many organisms (including humans). Proteins come in many different shapes and sizes. Some are globular (roughly spherical) in shape, whereas others form long, thin fibres. For example, the haemoglobin protein that carries oxygen in the blood is a globular protein, while collagen, found in the skin, is a fibrous protein.
4. A protein’s shape is critical to its function, and, many different types of chemical bonds may be important in maintaining this shape. Changes in temperature and pH, as well as the presence of certain chemicals, may disrupt a protein’s shape and cause it to lose functionality, a process known as denaturation.
5. Amino acids in simple aspect are the monomers which make up proteins. A polypeptide chain is formed when, a protein is made up of one or more linear chains of amino acids. In general, there are 20 different types of amino acids present in proteins.
6. Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (NH2) a carboxyl group {COOH} and a hydrogen atom.
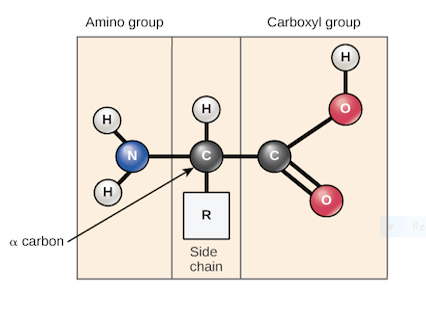
Fig 5: Basic structure of an aminoacidic, every amino acid structure also has another atom which may be present in a group or single atom that are bonded to the central atom, known as the R group, the R group is the one that determines the identity of the amino acid. For instance, if the R group is a hydrogen atom, then the amino acid is glycine etc.
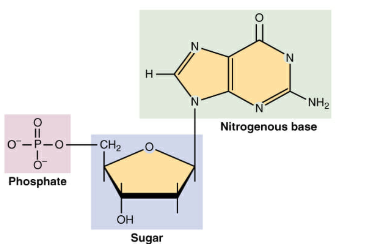
Fig 7: A nucleotide is made up of three parts: a phosphate group, a 5-carbon sugar, and a nitrogenous base
4. A nucleotide is an organic molecule that is the building block of DNA and RNA. They also have functions related to metabolism cell signalling, and enzyme reactions.
5. DNA which carries the genetic information stands for deoxyribonucleic acid, while RNA which is also involved in genetic transfer stands for ribonucleic acid. Although DNA and RNA both carry genetic information, there are quite a few differences between them. Of the two, RNA is more in nature than DNA, capable of performing enormous, diverse tasks in an organism, but DNA is more stable in nature and holds more complex information for longer periods of time.
6. DNA is present in the nucleus of a cell (Nuclear DNA) and in mitochondria (Mitochondrial DNA). It has two nucleotide strands which consist of its phosphate group, four nitrogen-containing nucleobases: adenine, cytosine, thymine and guanine five-carbon sugar (the stable 2-deoxyribose).
7. During transcription, a single-stranded, linear molecule RNA, is formed, which is complementary to DNA, helping to carry out the tasks that DNA wants it to do. Similar to DNA, RNA structure is composed of a five-carbon sugar that is less stable than Ribose and of its phosphate group, and four nitrogen-containing nucleobases: adenine, uracil (not thymine), and cytosine and guanine.
8. In both DNA and RNA molecules, the nucleobases are attached to their sugar-phosphate that forms the backbone of their structure. DNA has nucleotide strands on which the nucleobase is present, each nucleobase present on a nucleotide strand of DNA attaches to its partner nucleobase on a second strand: adenine always links to thymine, and cytosine links to guanine respectively. The base pairing on the opposite strands causes DNA's two strands to twist and wind around each other, resulting in a variety of shapes, such as the famous double helix (DNA's "relaxed" form), circles, and supercoils.
9. In RNA, uracil and adenine (not thymine) link together, while cytosine still links to guanine. Since RNA is a single stranded molecule unlike DNA which is double stranded, RNA folds in on itself to link up its nucleobases, though not all become paired. These folding subsequent forms the three-dimensional shapes, the most common one is the hairpin loop, that help determine what role the RNA molecule is to play.
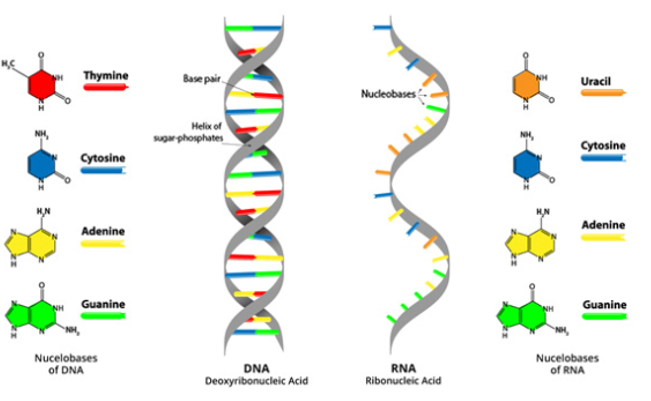 Fig 8: DNA consists of two strands, arranged in a double helix. These strands are made up of subunits called nucleotides. The bases in DNA are Thymine (‘T’) and Adenine (‘A’), Guanine (‘G’) and Cytosine (‘C’). RNA is a single strand, and the bases are Adenine and Uracil pair (A-U) Cytosine and Guanine pair (C-G) respectively.
Fig 8: DNA consists of two strands, arranged in a double helix. These strands are made up of subunits called nucleotides. The bases in DNA are Thymine (‘T’) and Adenine (‘A’), Guanine (‘G’) and Cytosine (‘C’). RNA is a single strand, and the bases are Adenine and Uracil pair (A-U) Cytosine and Guanine pair (C-G) respectively.
Comparison | DNA | RNA |
Full Name | Deoxyribonucleic Acid | Ribonucleic Acid |
Function | DNA replicates and helps in storing genetic information. The special feature is it is the blueprint for all genetic information contained within an organism is stored in DNA. | The main function of RNA is that it converts the genetic information present within DNA to a format useful to build proteins, and then moves it to ribosomal protein factories. |
Structure | DNA consists is double stranded, arranged in a double helix. These strands are made up of subunits called nucleotides. Each nucleotide contains a phosphate, a nitrogenous base and a 5-carbon sugar molecule. | RNA only is single stranded, but like DNA, strand is made up of nucleotides. RNA strands are shorter than DNA strands. RNA sometimes forms a secondary double helix structure, but only occasionally. |
Length | DNA is a much longer polymer than RNA. For example, a chromosome, is a single, long DNA molecule, when the molecule is opened it would be may centimetres long in length. | RNA molecules may vary in length, but are comparatively found to be much shorter than long DNA polymers. A large RNA molecule will be a few thousand base pairs in length. |
Sugar | The sugar in DNA is deoxyribose, which contains one less hydroxyl group than RNA’s ribose. | RNA contains ribose sugar molecules, without the hydroxyl modifications of deoxyribose. |
Bases | The bases in DNA are Adenine (‘A’), Thymine (‘T’), Guanine (‘G’) and Cytosine (‘C’). | RNA shares Adenine (‘A’), Guanine (‘G’) and Cytosine (‘C’) with DNA, but contains Uracil (‘U’) rather than Thymine. |
Base Pairs | Adenine and Thymine pair (A-T) Cytosine and Guanine pair (C-G) | Adenine and Uracil pair (A-U) Cytosine and Guanine pair (C-G) |
Location | DNA is found in the nucleus, in Mitochondria with a small amount of DNA is also present. | RNA forms in the nucleolus, and then moves to specialised regions of the cytoplasm depending on the type of RNA formed. |
Reactivity | DNA is a more stable molecule than RNA. The reason being the deoxyribose sugar, has one less oxygen-containing hydroxyl group, this makes DNA a useful molecule which has the task of keeping genetic information safe. | RNA, containing a ribose sugar, is more reactive than DNA and performs enormous tasks but is not stable in alkaline conditions. RNA’s larger helical grooves mean it is more easily affected by the attack of enzymes. |
Ultraviolet (UV) Sensitivity | DNA is easily affected when the molecule is exposed to ultraviolet light and can also be damaged. | RNA is comparatively more resistant to damage from UV light than DNA. |
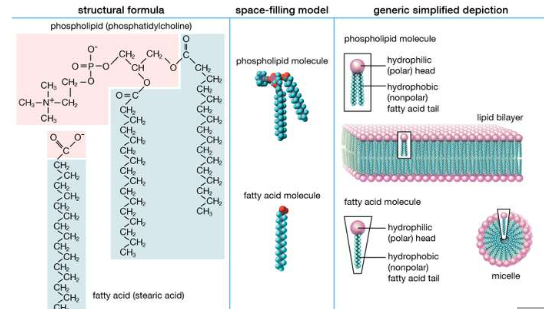
References: