Unit-1
Introduction to solid, liquid and gaseous fuels– Stoichiometry
1.1 Introduction to solid, liquid and gaseous fuels– Stoichiometry
Fuel is a substance which, when consumed on coming in contact and responding with Oxygen or air produces heat. Along these lines the substances delegated fuel should essentially contain one or a few of the burnable component’s carbon, hydrogen, sulphur, and so on during the time spent burning, the substance energy of fuel is changed over into heat energy.
To use the energy of fuel in most usable structure, it is needed to change the fuel from its one state to another, for example from strong to fluid or vaporous state, fluid to vaporous state, or from its compound energy to some other type of energy through single or numerous stages. Along these lines, the energy of powers can be used all the more viably and proficiently for different purposes.
Strong energizes are predominantly grouped into two classes, for example characteristic energizes, for example, wood, coal, and so forth and produced powers, for example, charcoal, coke, briquettes, and so on
Normally accessible coal
1) Peat: - It is the principal phase of development of coal far beneath the outside of earth. It contains a lot of dampness content up to 35%. Along these lines, it must be dried before use. Its normal calorific worth is 23000 kJ/kg.
2) Lignite: - It comes in the following phase of arrangement of coal after peat. It likewise has an enormous dampness substance might be up to 40%. On drying, it gets weak and changed over to pieces. It is viewed as better than Peat. Its calorific worth is around 25000 kJ/kg.
3) Bituminous Coal: - It is an unrivalled and normally accessible coal containing less dampness content however high carbon content. Its normal calorific worth is 33500kJ/kg. It is further of two sorts: Building up Bituminous coal and Non-Hardening Bituminous coal. Building up bituminous is a delicate coal however swells and structure pale mass on consuming. So consuming is hard to occur. It is valuable for creating coal gas. Non-Solidifying assortment is prevalent in consuming and creates less smoke. It is generally utilized in boilers for making steam as is called Steam Coke.
4) Anthracite Coal: - It is the last phase of coal arrangement and contains over 90% carbon. So it is likewise most unrivaled nature of normally accessible coal. Its consuming is smokeless and has a calorific estimation of roughly 36000 kJ/kg.
Artificially prepared coal
1) Wood charcoal: - It is set up by warming of wood with restricted stockpile of air at a temperature almost 3000C. It is utilized in metallurgical Enterprises.
2) Coke: - It is delivered by warming of normally accessible coal ceaselessly for two days without air. This cycle is referred to as carbonization of coal as the carbon content increments by eliminating of dampness and different gases. Because of high carbon content, it has a high calorific worth. On the off chance that coal is set up on warming at a temperature of 500-700oC, it stays delicate and called as Delicate Cake. It is utilized in home-grown applications. In the event that coke is set up at a higher temperature for example 900oC to 1100oC, it is called as hard coke. It is utilized in Vault heater for delivering Cast Iron.
3) Briquetted coal: - It is delivered by blending of finely ground coal with a coupling material like coal tar, earth and so on and afterward moulding it under tension. It has advantage that it doesn't slow down while dealing with thus forestalls loss of fuel.
4) Pummeled coal: - The name pounded is given to coal which is squashed to fine powder by beating machines. By and large low evaluated coal with high substance of debris is pummelled prior to utilizing in concrete and metallurgical Ventures.
5) Petrol coke: - It is the remaining of unrefined petroleum left in the wake of isolating the other helpful divisions like petroleum, diesel and so on in refining and refinement measures.
The different favourable circumstances and inconveniences of strong fills are given underneath
Advantages
(a) They are not difficult to ship.
(b) They are advantageous to store with no danger of unconstrained blast.
(c) Their expense of creation is low.
(d) They have moderate start temperature.
Disadvantages
(a) Their debris content is high.
(b) Their huge extent of warmth is squandered.
(c) They ignite with clinker development.
(d) Their ignition activity can't be controlled without any problem.
(e) Their expense of dealing with is high
These are the powers which are found in fluid state and simpler to deal with and use when contrasted with strong powers. The normally accessible fluid fuel is oil which isn't utilized straightforwardly as it is combination of various helpful substances. Along these lines prior to utilizing, it is refined by bubbling and build-up measure in processing plants refineries to isolate into various helpful substances like petroleum, lamp oil, fuel oils, greasing up oils, coal tar and so on Out of these some are utilized as powers, yet some like greasing up oil, coal tar are utilized in different applications, From our subject perspective the accompanying fluid energizes are significant:
1) Petroleum or gas: - It is the most light and most unstable fluid portion of oil fuel. It is refined at a lower temperature up to 2000C by bubbling of petrol, From that point breaking measure is utilized to get ready light petroleum. Petroleum is utilized as a fuel in all S.I. Motors. Oil is a fundamental normal fuel. It is a dull greenish earthy colored, gooey mineral oil, discovered somewhere down in earth's hull. It is for the most part made out of different hydrocarbons (like straight chain paraffin's, cycloparaffins or napthenes, olefins, and aromatics) along with modest quantity of natural mixes containing oxygen nitrogen and sulphur. The normal structure of unrefined petrol is C = 79.5 to 87.1%; H = 11.5 to 14.8%; S = 0.1 to 3.5%, N and O = 0.1 to 0.5%.
The straight run fuel is gotten either from refining of rough oil or by amalgamation. It contains some unfortunate unsaturated straight chain hydrocarbons and sulphur mixes. It has bubbling scope of 40-120oC. The, unsaturated hydrocarbons get oxidized and polymerized, in this way causing gum and slop arrangement on putting away. Then again, sulphur exacerbates lead to erosion of inside burning motor and simultaneously they antagonistically influence tetraethyl lead, which is for the most part added to fuel for better start properties.
The sulphur mixes from fuel are for the most part eliminated by treating it with a soluble arrangement sodium plumber. Olefins and shading matter of gas are normally taken out by permeating through 'Fuller's earth' which ingests especially just the tones and olefin. It is utilized in air-makes. It is likewise utilized as Engine fuel, in dry-cleaning and as a dissolvable. A portion of the qualities of an ideal fuel are the accompanying:
(a) It should be modest and promptly accessible.
(b) It should consume clean and produce no consumption, and so on burning.
(c) It should blend promptly with air and manage the cost of uniform complex dispersion, for example ought to effectively disintegrate.
(d) It should be thump safe.
(e) It ought to be pre-touch off without any problem.
(f) It should have a high calorific worth
Classification of Petroleum
The substance idea of unrefined petrol changes with the piece of the world in which it is found. They show up, notwithstanding, to be three head verities.
Paraffinic Base Sort Unrefined Petrol
This sort of petrol is for the most part made out of the soaked hydrocarbons from CH4 to C35 H72 and a tad bit of the napthenes and aromatics. The hydrocarbons from C18 H38 to C35 H72 are here and there called waxes.
Asphaltic Base Sort Unrefined Petrol
It contains essentially cycloparaffins or napthenes with more modest measure of paraffin's and fragrant hydrocarbons.
Blended Base Sort Unrefined Petrol
It contains both paraffinic and asphaltic hydrocarbons and are by and large wealthy in semi-strong waxes.
2) Lamp oil: - It is a heavier part and less unpredictable fuel and refined Lamp fuel oil is acquired between 180-250oC during fragmentary refining of unrefined petrol. It is utilized as an illuminant, fly motor fuel, farm truck fuel, and for getting ready lab gas. With the improvement of stream motor, lamp oil has gotten a material of far more prominent significance than it is utilized to be. At the point when lamp oil is utilized in homegrown machines, it is constantly disintegrated before burning. By utilizing a reasonable abundance of air it ignites with a smokeless blue fire.
Hefty Oil and its Attributes
It is a division acquired between 320-400oC during partial refining of rough Petrol. This oil on refractionation gives:
(a) Greasing up oils which are utilized as oils.
(b) Petrol jam (Vaseline) which is utilized as oils in meds and in beautifiers.
(c) Oils which are utilized as oils.
(d) Paraffin wax which is utilized in candles, boot shines, wax paper, tarpolin fabric and for electrical protection purposes.
3) Diesel Oils: - Diesel oils are the energizes which distillate on additional higher temperature up to 3700C. Diesel oils lie among Lamp fuel and greasing up oils while isolating from unrefined petrol. These are the powers which are economically utilized in all C.I. motors, Diesel Gensets, boilers and so on
The diesel fuel or gas oil is gotten between 250-320oC during the fragmentary refining of unrefined petrol. This oil for the most part contains 85% C. 12% H. Its calorific worth is around 11,000 kcal/kg. The appropriateness of a diesel fuel is dictated by its cetane esteem. Diesel fills comprise of longer hydrocarbons and have low estimations of debris, residue, water and sulphate.
The primary attributes of a diesel fuel are that it ought to effortlessly light underneath pressure temperature. The hydrocarbon particles in a diesel fuel ought to be quite far, the straight-chain ones, with a base admixture of sweet-smelling furthermore, side-chain hydrocarbon particles. It is utilized in diesel motors as warming oil and for breaking to get fuel.
4) Fuel Oils: - Fuel oil is like Diesel in explicit gravity and refining range, however their arrangement fluctuates in a wide reach. Or maybe fuel oils are utilized for mechanical reason. Diesel is likewise a sort of fuel oil.
The advantages and disadvantages of liquid fuels can be summarized as follows:
Advantages
(a) They have higher calorific worth per unit mass than strong fills.
(b) They consume without dust, debris, clinkers, and so forth
(c) Their terminating is simpler and furthermore fire can be stifled effectively by halting fluid fuel supply.
(d) They are not difficult to ship through lines.
(e) They can be put away inconclusively with no misfortune.
(f) They are spotless being used and financial to deal with.
(g) Loss of warmth in smokestack is extremely low because of more prominent neatness.
(h) They require less overabundance air for complete burning.
(I) They require less heater space for burning.
Disadvantages
(a) The expense of fluid fuel is moderately a lot higher when contrasted with strong fuel.
(b) Expensive extraordinary stockpiling tanks are needed for putting away fluid powers.
(c) There is a more serious danger of five risks, especially, in the event of profoundly inflammable and unpredictable fluid powers.
(d) They give awful smell.
(e) For proficient copying of fluid energizes, exceptionally built burners and showering contraption are required.
These are additionally normally accessible or misleadingly arranged. The flammable gas is accessible under the world's surface close to oil fields. It is a combination of methane, ethane and other like gases. Vaporous energizes are should have been packed for putting away in compartments and furthermore for powerful use. They are hard to deal with and require huge hefty compartments however their bit of leeway is that they promptly burst into flames and liberated from contaminations. So ignition is finished and furthermore contamination is less.
The vaporous fills, which are falsely set up from different powers, are ordered as:
1) Coal gas: - It is gotten via carbonization of coal as examined before in strong fills. It comprises primarily of hydrogen and a few hydrocarbons. Its calorific worth is around 25000 kJ/m3.
Coal Gas its Attributes
Coal gas is acquired when it is carbonized or warmed without air at about 1300oC in either coke broilers or gas-production counters. In gas making answer measure coal is taken care of in shut silica counters, which are then warmed to about 1300oC by consuming maker gas and air blend.
C + ½ O2 = CO + 29.5 kcal
Coal gas is a drab gas having a trademark scent. It is lighter than air and ignites with a long smoky fire. Its normal piece is : H2 = 47%, CH4 = 32%, CO = 7%, C2H2 = 2%, C2H4 = 3%, N2 = 4%, CO2 = 1% and rest = 4%. Its calorific worth is around 4900 kcal/m3.
It is utilized as (a) illuminate in urban communities and town, (b) a fuel, and (c) in metallurgical Tasks for giving diminishing air
Blast Furnace Gas and its Characteristics:
It is a result pipe gas acquired during the decrease of particle metal by coke in the impact heater. Its calorific worth is around 1000 kcal/m3. It contains around 20-25% carbon monoxide alongside CO2, N2, and so forth Around 1/3 of this gas is utilized for preheating air utilized in impact heater itself; while the leftover 2/third is accessible for use in boilers or subsequent to cleaning in gas motors. It is likewise utilized for consuming in an exceptional kind of ovens (called Cowper's oven) where the heater is preheated. This gas contains a lot of residue and is generally cleaned before use by dust pilgrims, twisters or electrolytic precipitators.
Water Gas and its Qualities:
Water gas is basically a combination of flammable gases CO and H2 with a little portion of non-ignitable gases. It is made by passing on the other hand steam and little air through a bed of intensely hot coal or coke kept up at around 900 to 1000Oc in a minister, which comprises of a steel vessel around 3 m wide and 4 m in tallness. It is fixed inside with fire-blocks. It has a cup and cone feeder at the top and an opening at the top for the exit of water gas. At the base, it is given gulf pipes for passing air and steam.
Reactions:
Provided steam responds with super-hot coke (or coal) at 900-1000oC to frame CO and H2.
C + H2O = CO + H2 – 29 kcal
C + O2 = CO2 + 97 kcal
Structure:
The normal structure of water gas is: H2 = 51%; CO = 41%; N2 = 4%; CO2 = 4%. Its calorific worth is around 2800 kcal/m3.
Uses
It is utilized as (a) a wellspring of hydrogen gas, (b) an enlightening gas, and (c) a fuel gas.
2) Liquefied petrol gas: It is set up from combustible gas by disconnecting lighter hydrocarbons for instance Propane and butane. It is liquefied and put away of chambers under high strain. Simply under high tension, a gas can be consolidated at barometrical temperature. Due to this a colossal measure of gas devours less space in chamber. LPG is routinely used as cooking gas and besides in gas engines and other Mechanical cycles.
3) Producer gas: It is obtained by midway start of coal inside seeing air and steam sway. It is used in glass dissolving measure. Producer gas is essentially a mix of burnable gases carbon monoxide and hydrogen related with non-ignitable gases N2, CO2, etcIt is set up by passing air mixed in with little steam (about 0.35 kg/kg of coal) over a searing coal or coke bed kept up at about 1100oC in a remarkable reactor called gas producer. It contains a steel vessel around 3 m in broadness and 4 m in stature. The vessel is fixed inside with fire blocks. It is outfitted with a cup and cone feeder at the top and a side opening for the exit of maker gas. At the base it has an inlet for passing air and steam. The makers at the base are furthermore outfitted with an exit for the trash outlined.
Reactions
The gas creation responses can be separated into four zones as follows:
Ash Zone
The least zone comprises of for the most part of debris, and along these lines, it is known as debris zone.
Ignition Zone
The zone close to the debris zone is known as oxidation or ignition zone. Here the carbon consumes and shapes CO and CO2. The temperature of this zone is about 1100oC. The accompanying responses happen.
C+O2=CO2 + 94 Kcal
C + ½ O2 = CO + 29.5 kcal
Reduction Zone
Here carbon dioxide and steam consolidates with super-hot carbon and Frees free hydrogen and carbon monoxide. The responses are:
CO2 + C = 2CO – 94 kcal
C + H2O = CO + H2 + 29 kcal
C + 2H2O = CO2 + 2H2 – 19 kcal
All these decrease responses are endothermic, thus, the temperature in the decrease zone tumbles to 1000oC.
Distillation zone
In this zone (400 – 800oC) the approaching coal is warmed by active gases by giving reasonable warmth to the coal. The warmth given by the gases and warmth transmitted from the decrease zone serves to distillate the fuel consequently unpredictable matter of coal is added to the active gas.
Composition
The normal organization of maker gas is CO = 22.3%, H2 = 8.12%; N2 = 52.55%; CO2 = 3%. Its calorific worth is around 1300 kcal/m3.
Uses
It is modest, clean and effectively preparable gas and is utilized (I) for warming open-hearth heaters (in steel and glass fabricate), mute heaters, answers (utilized in coke and coal gas make), and so forth and (iii) as a diminishing specialist in metallurgical tasks.
Gaseous fuels occur in nature, besides being manufactured from solid and liquid fuels.
The advantages and disadvantages of gaseous fuels are given below:
Advantages
Gaseous fuels due to erase and flexibility of their applications possess the Following advantages over solid or liquid fuels:
(a) They can be passed on effectively through pipelines to the real spot of Need accordingly dispensing with difficult work in transportation.
(b) They can be lit calm.
(c) They have high warmth substance and consequently help us in having higher temperatures.
(d) They can be pre-warmed by the warmth of hot waste gases, in this way influencing economy in warmth.
(e) Their ignition can promptly by controlled for change popular like oxidizing or decreasing air, length fire, temperature, and so on
(f) They are spotless being used.
(g) They don't need any extraordinary burner.
(h) They consume with no shoot, or smoke and cinders.
(I) They are liberated from pollutions found in strong and fluid fills.
Disadvantages
(a) Extremely enormous capacity tanks are required.
(b) They are exceptionally inflammable, so odds of fire risks in their utilization are high.
Key Takeaways:
The essential ignition cycle can be depicted by the fuel (the hydrocarbon) in addition to oxidizer (air or oxygen) called the Reactants, which go through a chemical process while delivering heat to form the product of combustion such that mass is conserved.
In the least complex burning cycle, known as Stoichiometric combustion, all the carbon in the fuel structures carbon dioxide (CO2) and all the hydrogen structures water (H2O) in the items, in this manner we can compose the chemical reaction as follows:
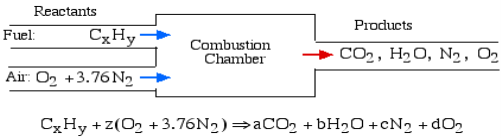
Where z is known as the stoichiometric coefficient for the oxidizer (air) Note that this response yields five questions: z, a, b, c, d, subsequently we need five conditions to tackle. Stoichiometric burning accepts that no abundance oxygen exists in the items, consequently d = 0. We get the other four conditions from adjusting the quantity of particles of every component in the reactants (carbon, hydrogen, oxygen and nitrogen) with the quantity of atoms of those components in the items. This implies that no molecules are demolished or lost in a burning response.
Element | Amount in reactants | = | Amount in Products | Reduced equation |
Carbon (C) | X |
| a | a = x |
Hydrogen (H) | Y |
| 2b | b = y/2 |
Oxygen (O) | 2z |
| 2a+b | z = a + b/2 |
Nitrogen (N) | 2(3.76)z |
| 2c | c = 3.76z |
Key Takeaways:
Note that the water formed could be in the fume or fluid stage, contingent upon the temperature and pressing factor of the ignition items.
As an illustration think about the stoichiometric burning of methane (CH4) in air. Likening the molar coefficients of the reactants and the items we get:
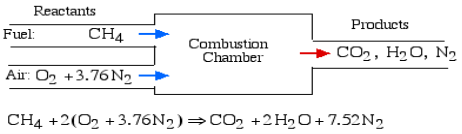
The First Law Analysis of Combustion:
The fundamental motivation behind ignition is to deliver heat through a difference in enthalpy from the reactants to the items. From the first Law condition in a control volume, ignoring kinetic and potential energy changes and expecting no work is done, we have:
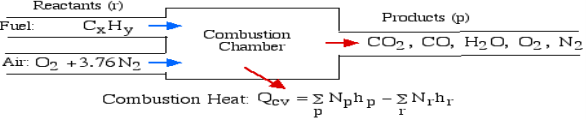
Where the summations are assumed control over all the items (p) and the reactants (r). N alludes to the quantity of moles of every part and h [kJ/kmol] alludes to the molar enthalpy of every segment.
For example, rethink the total ignition of Methane (CH4) with hypothetical air:
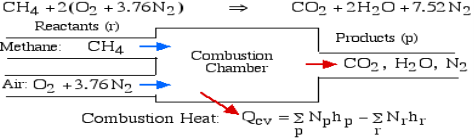
Notice that in the reactants and the results of the above model we have essential components O2 and N2 just as mixes CH4, CO2, and H2O. At the point when the compound is shaped then the enthalpy change is known as the Enthalpy of Development, signified hfo, and for our model:
Substance | Formula | hfo [kJ/kmol] |
Carbon dioxide | CO2(g) | -393,520 |
Water Vapor | H2O(g) | -241,820 |
Water | H2O(l) | -285,820 |
Methane | CH4(g) | -74,850 |
Where (g) alludes to gas and (l) alludes to fluid. The negative sign implies that the cycle is Exothermic, for example heat is radiated when the compound is framed. Note that the enthalpy of development of fundamental components O2 and N2 is zero.
Consider first the case in which there is adequate heat transfer such that both the reactants and the items are at 25°C and 1 atom pressure, and that the water item is fluid. Since there is no reasonable enthalpy change the energy condition becomes:
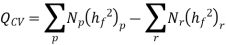
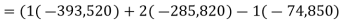
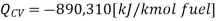
On a unit basis mass:
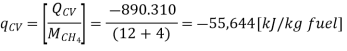
This heat (Qcv) is known as the Enthalpy of Ignition or the heating Estimation of the fuel. In the event that the items contain fluid water, at that point it is the Higher heating value (as in our example), in any case in the event that the item contains water fume, at that point it is the Lower heating value of the fuel. The enthalpy of combustion is the biggest amount of heat that can be delivered by a given fuel.
The precise definition of enthalpy (H) is the sum of the internal energy (U) plus the product of pressure (P) and volume (V). In symbols, this is:
H = U + PV
A change in enthalpy (∆H) is therefore:
∆H = ∆U + ∆P∆V
Where the delta symbol (∆) means “change in.” In practice, the pressure is held constant and the above equation is better shown as:
∆H = ∆U + P∆V
However, for a constant pressure, the change in enthalpy is simply the heat (q) transferred:
∆H = q
If (q) is positive, the reaction is endothermic (i.e., absorbs heat from its surroundings), and if it is negative, the reaction is exothermic (i.e., releases heat into its surroundings). Enthalpy has units of kJ/mol or J/mol, or in general, energy/mass. The equations above are really related to the physics of heat flow and energy: thermodynamics.
Simple Enthalpy Change Calculation:
The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants. If you know these quantities, use the following formula to work out the overall change:
∆H = Hproducts − Hreactants
The addition of a sodium ion to a chloride ion to form sodium chloride is an example of a reaction you can calculate this way. Ionic sodium has an enthalpy of −239.7 kJ/mol, and chloride ion has enthalpy −167.4 kJ/mol.
Key Takeaways:
Sodium chloride (table salt) has an enthalpy of −411 kJ/mol. Inserting these values gives:
∆H = −411 kJ/mol – (−239.7 kJ/mol −167.4 kJ/mol)
= −411 kJ/mol – (−407.1 kJ/mol)
= −411 kJ/mol + 407.1 kJ/mol = −3.9 kJ/mol
So the formation of salt releases almost 4 kJ of energy per mole.
At the point when a combustion reaction takes place energy is delivered to the ignition items. On the off chance that no heat is lost in this cycle, the temperature of the burning items is known as the "Adiabatic Fire Temperature.
For an combustion cycle that happens adiabatically with no shaft work, the temperature of the items is alluded to as the adiabatic fire temperature. This is the greatest temperature that can be accomplished for given reactants. heat transfer, incomplete combustion, and separation all outcome in lower temperature. The most extreme adiabatic flame temperature for a given fuel and oxidizer mix happens with a stoichiometric combination (right extents to such an extent that all fuel and all oxidizer are burned-through). The measure of abundance air can be custom fitted as a component of the plan to control the adiabatic fire temperature. The significant distance between present temperatures in a gas turbine engine and the most extreme adiabatic flame temperature at stoichiometric conditions compressor exit temperature of 12000F (922 K).
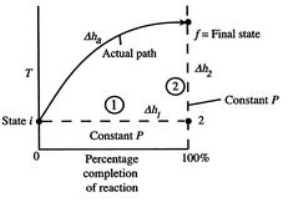
Figure 1: Schematic of adiabatic flame temperature
An initial perspective on the idea of adiabatic flame temperature is given by inspecting two responding gases, at a given pressing factor, and asking what the end temperature is. The cycle is indicated schematically in Figure 1, where temperature is plotted versus the percentage completion of the reaction. The underlying state is i and the last state is f with the final state at a higher temperature than the initial state. The strong line in the figure shows a portrayal of the ''actual'' process.
To perceive how we would show up at the final completion state the dashed lines break the condition of reaction change into two sections. Process (1) is reaction at consistent T and P. To complete such a cycle, we would have to extract heat. Assume the aggregate sum of heat extraction per unit mass is q1. The connection between the enthalpy changes in process (1) is
h2- h1= -q1= (hof) unit mass
Where q1is the heat of reaction.''
For Process (2), we put this amount back into the products to raise their temperature to the final level. For this process,
hf – h2 = q1
Or if we can approximate the specific heat as constant (using some appropriate average value).
CP.avg(Tf – T2) = q1
For the overall process there is no work done and no heat exchanged so that the difference in enthalpy between initial and final states is zero:
∆h1 + ∆h2 = ∆Hadiabatic = 0
The temperature change during this second process is therefore given by (approximately).
(Tf – T2) = q1 / Cp.avg = (hof) unit mass / Cp.avg
The estimation of the adiabatic flame temperature given in Condition (15.5) is for 100% finish of the reaction. In reality, as the temperature increases, the tendency is for the degree of reaction to be less than 100%. For example, for the burning of hydrogen and oxygen, at high temperatures the combustion product (water) separates once more into the less difficult basic reactants. The degree of reaction is in this manner itself a component of temperature that need to be computed. We utilized this thought in discussing the stoichiometric ramjet, when we said that the greatest temperature was autonomous of flight Mach number and subsequently of inlet stagnation temperature. It is also to be emphasized that the idea of a constant (average) specific heat, Cp average is for illustration and not inherently part of the definition of adiabatic flame temperature.
A model calculation of adiabatic flame temperature is furnished by the combustion of liquid octane at with 400% hypothetical air. The response is
C8H18(l) + 12.3 O2 + 12.5(3.76N2) + 3[12.5O2 + 12.5(3.76N2)] → 8CO2 + 9H2O(g) + 37.5O2 + 188N2
For an adiabatic process
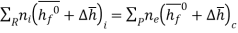 where
where 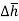 at adiabatic flame temperature
at adiabatic flame temperature
We can again think of the general process in steps:
1. Bring reactants to 250C [the term(∆h)i ] from the initial temperature, using whatever heat transfer, qa is required. In this model we needn't bother with step (i) since we are now at the reference temperature.
2. Reaction at 250c [the term(hof) reactants-products]. There will be some heat transfer in this progression, qb out of the combustor.
3. Put back warmth qa+qb into the results of ignition. The subsequent temperature is the adiabatic flame temperature.
In the present case
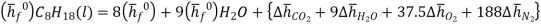
We can look at the terms in the SFEE independently, beginning with the heat of formation terms, and monitoring units:
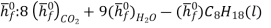
= 8 k mole (-393,522 kJ /k mole) + 9 k mole (-241,827 kJ/k mole)
= - 1 k mole (-249,952 KJ/k mole)
= - 5.075 X 106 kJ
The exit state at the adiabatic flame temperature is determined by:
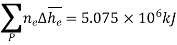
We find the adiabatic flame temperature in three ways:
An approximate solution using an average value of Cp
A more precise one utilizing the organized development of Cp with temperature,
Key Takeaways:
We have identified three criteria for whether a given reaction will occur spontaneously: ΔSuniv > 0, ΔGsys < 0, and the relative magnitude of the reaction quotient Q versus the equilibrium constant K. Recall that if Q < K, then the reaction proceeds spontaneously to the right as written, resulting in the net conversion of reactants to products. Conversely, if Q > K, then the reaction proceeds spontaneously to the left as written, resulting in the net conversion of products to reactants. If Q = K, then the system is at equilibrium, and no net reaction occurs. Table 1 summarizes these criteria and their relative values for spontaneous, nonspontaneous, and equilibrium processes.
Table Criteria for the Spontaneity of a Process | ||
Spontaneous | Equilibrium | Nonspontaneous* |
*Spontaneous in the reverse direction. | ||
ΔSuniv > 0 | ΔSuniv = 0 | ΔSuniv < 0 |
ΔGsys < 0 | ΔGsys = 0 | ΔGsys > 0 |
Q < K | Q = K |
|
Because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising indeed if they were not related. In this section, we explore the relationship between the standard free energy of reaction (ΔG°) and the equilibrium constant (K).
Because ΔH° and ΔS° determine the magnitude of ΔG° and because K is a measure of the ratio of the concentrations of products to the concentrations of reactants, we should be able to express K in terms of ΔG° and vice versa. "Free Energy", ΔG is equal to the maximum amount of work a system can perform on its surroundings while undergoing a spontaneous change. For a reversible process that does not involve external work, we can express the change in free energy in terms of volume, pressure, entropy, and temperature, thereby eliminating ΔH from the equation for ΔG. The general relationship can be shown as follow
ΔG=VΔP-SΔT…..1
If a reaction is carried out at constant temperature (ΔT = 0), then Equation 1 simplifies to
ΔG=VΔP…..2
Under normal conditions, the pressure dependence of free energy is not important for solids and liquids because of their small molar volumes. For reactions that involve gases, however, the effect of pressure on free energy is very important.
Assuming ideal gas behaviour, we can replace the V in Equation 2 by nRT/P (where n is the number of moles of gas and R is the ideal gas constant) and express ΔGin terms of the initial and final pressures (Pi and Pf , respectively):
ΔG = (nRT / P) ΔP = n RT (ΔP / P) = n RT in (Pf / Pi)……3
If the initial state is the standard state with Pi = 1atm then the change in free energy of a substance when going from the standard state to any other state with a pressure P can be written as follows:
G−G°=nRTlnP…….4
This can be rearranged as follows:
G=G°+nRTlnP…..5
As you will soon discover, Equation 5allows us to relate ΔG° and Kp. Any relationship that is true for Kp must also be true for K because Kp and K are simply different ways of expressing the equilibrium constant using different units.
Let’s consider the following hypothetical reaction, in which all the reactants and the products are ideal gases and the lowercase letters correspond to the stoichiometric coefficients for the various species:
aA+bB⇌cC+dD……..6
Because the free-energy change for a reaction is the difference between the sum of the free energies of the products and the reactants, we can write the following expression for ΔG:
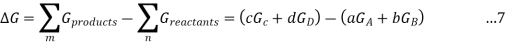
Substituting Equation 5 for each term into Equation 7,
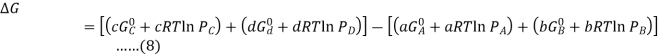
Combining terms gives the following relationship between ΔG and the reaction quotient Q:
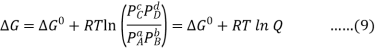
Where ΔG° indicates that all reactants and products are in their standard states. For gases at equilibrium (Q=KpQ=Kp), and as you’ve learned in this chapter, ΔG = 0 for a system at equilibrium. Therefore, we can describe the relationship between ΔG° and Kp for gases as follows:
0=ΔG°+RTlnKp……10
ΔG°=−RTlnKp……...11
Key Takeaways:
If the products and reactants are in their standard states and ΔG°< 0, then Kp > 1, and products are favoured over reactants when the reaction is at equilibrium. Conversely, if ΔG°> 0, then Kp < 1, and reactants are favoured over products when the reaction is at equilibrium. If ΔG° = 0, then Kp=1and neither reactants nor products are favoured when the reaction is at equilibrium.
Reference
1) Engineering thermodynamics P.K nag
2) I.C engineering =m.l mathur
3) https://www.physicsforums.com/attachments/flow-calculations-pdf.20219/
4) https://onlinelibrary.wiley.com/doi/pdf/10.1002/0471743984.vse7245