UNIT 1
Quantum Chemistry and Spectroscopy
Postulates of quantum mechanics:
Postulate 01
The wave functions are responsible for the determination of the properties of quantum mechanical system.
Postulate 02
The wave function ψ obeys the time-dependent Schrodinger equation
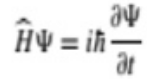
Where,
H is the Hamiltonian operator of the system
Postulate 03
This postulate state that for each operator there is only one variable and for each variable there is only one mathematical operator.
Postulate 04
If a mechanical variable A is measured without experimental error, the only possible outcomes of the measurement are the eigen values of the operator A that corresponds to A
The expectation value for the error-free measurement of a mechanical variable A is given by the formula
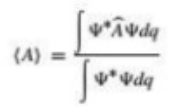
Where,
A is operator corresponding to A
Ψ= ψ(q,t)
Postulate 05
Determination and measurement of the state of a function.
The Schrodinger equation is a linear partial differential equation that describes the wave function or state function of a quantum-mechanical system. Schrodinger wave equation is a mathematical expression describing the energy and position of the electron in space and time, taking into account the matter wave nature of the electron inside an atom.
It is based on three considerations. They are;
Classical Plane Wave Equation
A wave is a disturbance of a physical quantity undergoing simple harmonic motion or oscillations about its place. The disturbance gets passed on to its neighbors in a sinusoidal form.
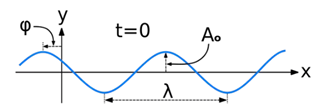
The equation for the wave is a second-order partial differential equation of a scalar variable in terms of one or more space variable and time variable. The one-dimensional wave equation is-
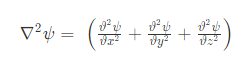
The amplitude (y) for example of a plane progressive sinusoidal wave is given by:
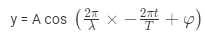
where, A is the maximum amplitude, T is the period and φ is the phase difference of the wave if any and t is the time in seconds. For a standing wave-
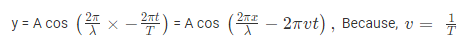
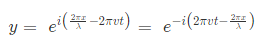
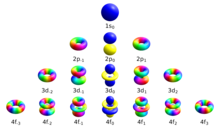
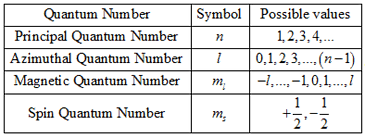
The quantum number provides the information about the spatial distribution of electrons. Schrodinger approach requires three different quantum numbers to specify a wave function for electrons.
The principal quantum number (n): They are denoted by the symbol n. Principal quantum number describe the distance between nucleus and electrons. The larger the value of n the greater is the distance between electron and nucleus. The value n=1 denotes the very innermost shell of an atom. So that it can be stated that principal quantum number cannot be zero or negative.
The Azimuthal quantum number: They are denoted by the symbol l. One of three quantum numbers that describe the shape of the region of space by an electron. The value of l describes the shape of the region of space occupied by the electron. The value of l depends on the value of n and that can range from 0 to n-1.
l= 0, 1, 2, 3, …….(n-1)
The Magnetic quantum number: The total number of orbitals in a subshell and the orientation of these orbitals are determined by the magnetic quantum number. It is denoted by the symbol ‘ml’. This number yields the projection of the angular momentum corresponding to the orbital along a given axis.
The amount of energy transmitted or absorbed by a solution is proportional to the solutions molar absorptive and the concentration of solute. In simple terms, a more concentrated solution absorbs more light than a more dilute solution does. Mathematically this can be expressed by
A=ε/C
where,
A = absorption,
Ε = molar attenuation coefficient
I = path length
C = concentration of the solution
Derivation of the Beer-Lambert law
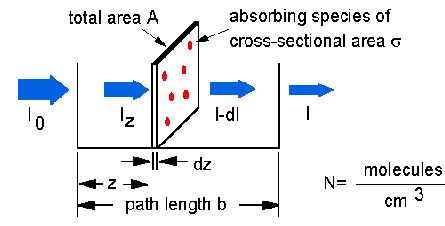
The Beer-Lambert law can be derived from an approximation for the absorption coefficient for a molecule by approximating the molecule by an opaque disk whose cross-sectional area 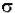 represents the effective area seen by a photon of frequency w. If the frequency of the light is far from resonance, the area is approximately 0, and if w is close to resonance the area is a maximum. Taking an infinitesimal slab, dz, of sample:
represents the effective area seen by a photon of frequency w. If the frequency of the light is far from resonance, the area is approximately 0, and if w is close to resonance the area is a maximum. Taking an infinitesimal slab, dz, of sample:
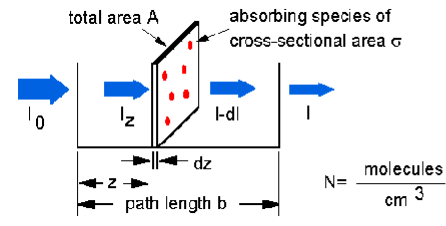
Io is the intensity entering the sample at z=0, Iz is the intensity entering the infinitesimal slab at z, dI is the intensity absorbed in the slab, and I is the intensity of light leaving the sample. Then, the total opaque area on the slab due to the absorbers is 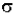 * N * A * dz. Then, the fraction of photons absorbed will be
* N * A * dz. Then, the fraction of photons absorbed will be 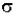 * N * A * dz / A so,
* N * A * dz / A so,
dI / Iz = - 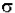 * N * dz
* N * dz
Integrating this equation from z = 0 to z = b gives:
In (I) – ln (Io) = - 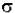 * N * b
* N * b
- ln (I / Io) = 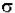 * N * b.
* N * b.
Since N (molecules/cm3) * (1 mole / 6.023x1023 molecules) * 1000 cm3 / liter = c (moles/liter) and 2.303 * log(x) = ln(x) then
- log (I / Io) = 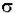 * (6.023x1020 / 2.303) * c * b
* (6.023x1020 / 2.303) * c * b
- log (I / Io) = A = 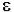 * b * c
* b * c
Where,
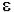 =
= 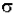 * (6.023x1020 / 2.303) =
* (6.023x1020 / 2.303) = 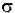 * 2.61x1020
* 2.61x1020
Ultraviolet and Visible Spectroscopy helps ion the determination of the absorbance spectra of the compound in the solution. The absorbance of light energy or the electromagnetic radiations are observed. This is responsible for the exciting the electron from the ground state to the first singlet excited state of a compound. The UV-Visible region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm.
Monochromator used to measure the wavelength of the light. Monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has diode array detectors that allow the instrument to simultaneously detect the absorbance at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
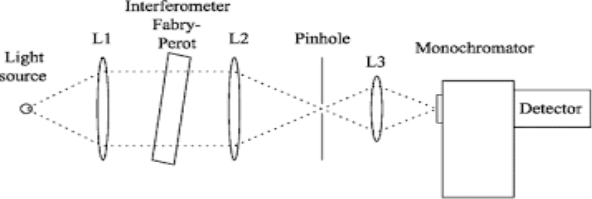
Applications:
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High performance liquid chromatography.
Ultraviolet- Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of water.
Chromophores:
It is used in the denotation of some structural features of which gives color to compound. E.g. Nitro group is a chromophore because its presence in a compound gives yellow color to the compound. But these days the term chromophore is used in a much broader sense which may be defined as “any group which exhibit absorption of electromagnetic radiation in a visible or ultra-visible region “It may or may not impart any color to the compound. Some of the important chromophores are: ethylene, acetylene, carbonyls, acids, esters and nitrile groups etc. There are two types of chromophores:
1. Chromophores in which the groups have π electrons undergo π-π* transitions. For examples:-ethylenes, acetylenes etc.
2. Chromophores having both π- electrons and n (non-bonding) electrons undergo two types of transitions. i.e., π-π* and n-π*, for examples: - carbonyls, nitriles and nitro compounds etc.
Applications on quantitative analysis:
1 This is used in clinical and pre-clinical application in cancer.
2 This is used in the investigation of platelets quality.
3 Quantitative monitoring of an activated sludge reactor using on-line UC-Visible and near-infrared spectroscopy.
4 The UV spectroscopy is used in the evaluation of pearl quality.
The increase in conjugation in a molecule, results in higher delocalization of electrons.
This delocalization effect the molecular orbital.
That results in the decrease of transition energy and thus a red or bathochromic effect. (The change in spectral band position in the absorption, reflectance, transmittance, of a molecule to longer wavelength. Because the red color in the visible spectrum has a longer wavelength than most other colors, the effect is also commonly called a red shift.)
The molar absorptivity will increase in this case and better quantitative analysis will be achieved.
In case of introduction of more un-conjugated double bonds, the molar absorptivity increase as well depending on the number of the double bonds.
Compound | Structure | On reacting with Br2 | Thermodynamic Stabilization |
1,3,5,7-Cyclooctatetraene |
| Addition 0oC | Slight |
Benzene |
| Substitution | Large |
Pyridine |
| Substitution | Large |
Furan |
| Substitution | Moderate |
Pyrrole |
| Substitution | Moderate |
Factors required for aromaticity:
A planar cycle of sp2 hybridized atoms, the p-orbital of which are oriented parallel to each other. These overlapping p-orbital generate an array of π-molecular orbital.
These π-orbital are occupied by 4n+2 electrons (where n is an integer or zero). This requirement is known as The Huckel Rule.
Rotational Spectroscopy: Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Applications:
Vibrational Spectroscopy: The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Taking the molecule A-B in consideration then the one get partial negative while other will be partial positive charge. There are two different type of vibration that is Coupled vibration and De-Coupled Vibrations. The change in the relative position of 2 atoms is called as the coupled vibration while de-coupled vibration means 1 atom get fixed at a single position instead the other atom is in rotation. The vibration leads to the expansion and contraction of the atoms in the molecule. The change in the dipole movement due to the vibration then only those molecule exhibits vibrational spectroscopy.
Molecular vibration: The periodic motion of atom of molecule relative to each other, such that the centre of mass of the molecule remains unchanged. There are six types of molecular vibration modes, which are:
1 Symmetric Stretching
2 Asymmetric Stretching
3 Wagging
4 Twisting
5 Scissoring
6 Rocking
Selection Rule: On the irradiation of the sample with infrared electromagnetic radiation then its frequency can be measured by the Infrared spectroscopy. It is a technique used to study the vibrations between atoms because atomic vibrational excitations occur in the infrared region of the electromagnetic spectrum. The bonds between atoms can be thought of as a spring connecting two masses. In the spring-mass analogy the moving system can be approximated by a simple harmonic oscillator. The frequency oscillation is proportional to √k/m
Where,
k is the spring constant
m is the mass of the object.
Applications:
Rotational Spectroscopy:
Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Applications:
Vibrational Spectroscopy/IR Spectroscopy:
The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Taking the molecule A-B in consideration then the one get partial negative while other will be partial positive charge. There are two different type of vibration that is Coupled vibration and De-Coupled Vibrations. The change in the relative position of 2 atoms is called as the coupled vibration while de-coupled vibration means 1 atom get fixed at a single position instead the other atom is in rotation. The vibration leads to the expansion and contraction of the atoms in the molecule. The change in the dipole movement due to the vibration then only those molecule exhibits vibrational spectroscopy.
Applications: