UNIT 4
Corrosion
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
4.2 Galvanic series
Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection of metals having minimal tendency to attract galvinacally, or will indicate the need or degree of protection to be applied to lessen the expected potential interactions. In general, the further apart the materials are in the galvanic series, the higher the risk of galvanic corrosion, which should be prevented by design. Conversely, the farther one metal is from another, the greater the corrosion will be. However, the series does not provide any information on the rate of galvanic corrosion and thus serves as a basic qualitative guide only.
Chemical Corrosion: The reaction of metal with water vapour or gas at high temperature causes the metal to corrode chemically. This is the redox process in which the electron of the metal are passed directly to the substance in the environment. The metal corrodes generally in the metal which is in higher contact with water.
 3Fe + 4H2O Fe3O4 + 4H2
3Fe + 4H2O Fe3O4 + 4H2
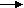 3Fe + 2O2 Fe3O4
3Fe + 2O2 Fe3O4
Electrochemical Corrosion: Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by teh elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
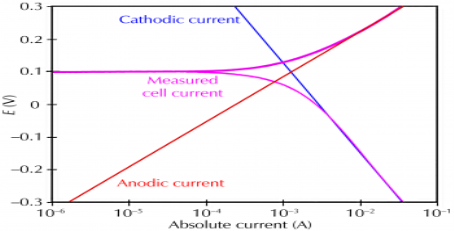
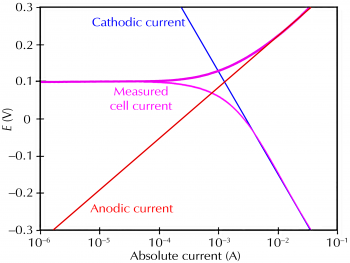
Corrosion process showing the anodic and cathodic component of current
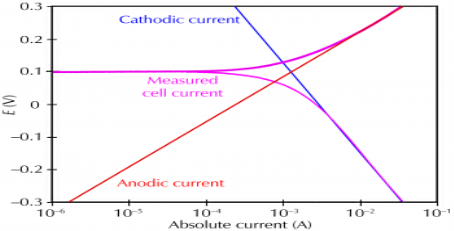
Mechanism of dry corrosion due to O2 gas there are 4 types: -
Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
The uneven supply of oxygen to the same metal component leads to the formation of oxygen concentration cells that are called as the differential aeration theory of corrosion. It is the type of electrochemical corrosion that affects the metals such as steel and iron. The less oxygenated part behaves anodic while the more oxygenated part cathodic. Since cathodic reactions involve consumption of oxygen, the more oxygenated part behaves cathodic and less oxygenated pan behaves anodic. The reaction occurs because oppositely charged electrons flow between the smaller anode and larger cathode. Positively charged cations meeting negatively charged anions forming corrosion product and a resulting pit in the metal, otherwise known as pitting corrosion. In a gutter, pipe, tank or similar the anode is just below the waterline. This is where the oxidation occurs, corrosion product forms and a pit develops weakening the metal.
4.6 Stress corrosion (caustic embrittlement in boilers)
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments. The chemical environment that causes SCC for a given alloy is often one which is only mildly corrosive to the metal. Hence, metal parts with severe SCC can appear bright and shiny, while being filled with microscopic cracks. This factor makes it common for SCC to go undetected prior to failure. SCC often progresses rapidly, and is more common among alloys than pure metals. The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.
Position of metal in galvanic series.
If position is higher in galvanic series then it carrode faster
While for 2 metal the difference between them shows the corrosion ratio.
2. Potential Difference
If the difference at the electrode potential between two metal is high then the rate of corrosion would be also high while vice versa for lesser difference.
3. Purity of metal
Corrosion never took place in pure metals. While if metal itself has a impurity then galvanic cell set up easily which intend increases the rate of corrosion.
4. Relative areas of cathode and anode parts
Rate of corrosion is directly depends on the area of cathode and inversely depends on the area of anode. If the area of cathode is larger then there is more demand of electrons while in the smaller anode area the corrosion took place very fast.
5. Nature of corrosion Product
Metal oxide film is formed on the surface of metal by corrosion due to oxygen. The formed film would be stable, unstable, volatile.
6. Temperature
At high temperature the rate of corrosion increases as because there is a consistent increase in the ionization and mobility difference rate while in some cases rate of corrosion decreases at high temperature as the solubility of O2 gas increases.
7. Presence of moisture
The rate of corrosion decreases in dry while increases in presence of moisture. Moisture act as the solvent for setting up of electrochemical corrosion.
8. Effect of pH
Rate of corrosion is high at acidic pH due to the evolution of H2 gas at cathode.
9. Concentration of electrolytes
This is also called as the Oxygen concentration cell. The rate of corrosion would be directly depends on the supply of oxygen on air.
10. Over Voltage
The difference between the actual value and theoretical value of decomposition potential of electrode.
Hot dipping processes:
1) Galvanizing:
Method:
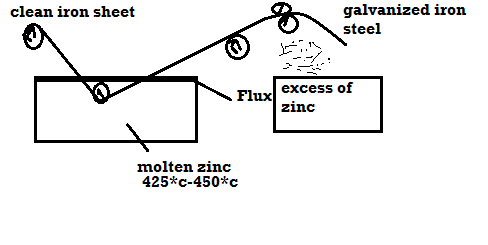
Applications: -
Tinning: -
Method: -
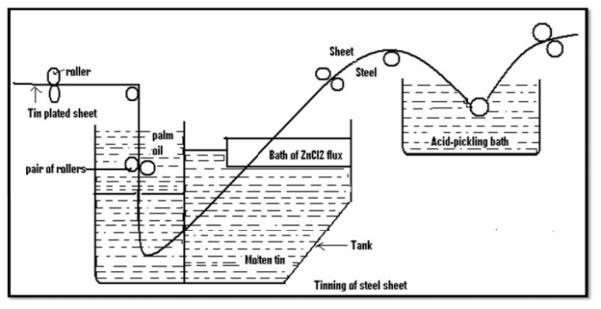
It is a chemical compound that decreases the corrosion rate of a material when added to a liquid or gas. Chemical substance which is present in the corrosion system at a suitable concentration decreases the corrosion rate, without significantly changing the concentration of any corrosive agent.
The nature of the corrosive inhibitor depends on –
(i) The material being protected
(ii) The corrosive agent to be neutralized
Sacrificial anodic protection
The metal surface can be protected from the corrosion by connecting it wire to a more anodic metal. The sacrifice of this more anodic metal to save the metal form corrosion is called as the Sacrificial Anode. The most common metal used for this purpose are Mg, Zn, Al etc.
Applications:
(i) The underground cable and pipeline protection from soil erosion.
(ii) Ships and boat protection from marine corrosion.
Prevention of rusty water by inserting Mg sheets or rods into domestic water boiler or tanks.
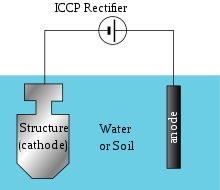
Impressed current method
This is the type of corrosion protection which consist of sacrificial anodes that is connected to an external power source. The external power source is DC power supply, that provides the sufficient current to drive electrochemical reaction required for the cathodic protection to occur.