Unit – 2
Concept of plastic deformation of metals
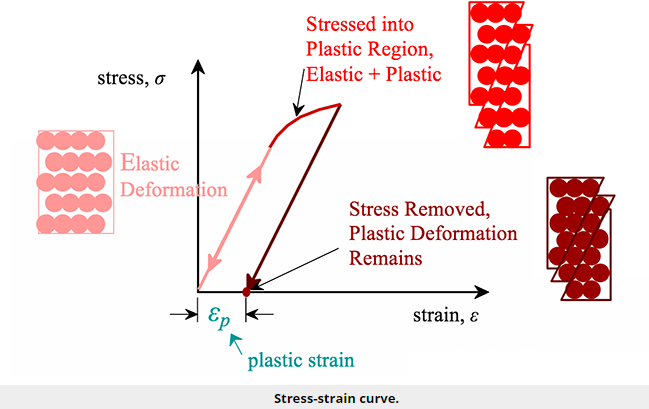
For most metallic materials, the elastic deformation region is relatively small. At some point, the strain is no longer proportional to the applied stress. At this point, bonds with original atom neighbours start to break and reform with a new group of atoms. When this occurs and the stress is relieved, the material will no longer return to its original form, i.e., the deformation is permanent and nonrecoverable. The material has now moved into the region referred to as plastic deformation. In practice, it is difficult to identify the exact point at which a material moves from the elastic region to the plastic region. As shown in the figure below, a parallel line offset by 0.002 strain is drawn. Where that line intercepts the stress-strain curve is identified as the yield strength. The yield strength is equal to the stress at which noticeable plastic deformation has occurred.
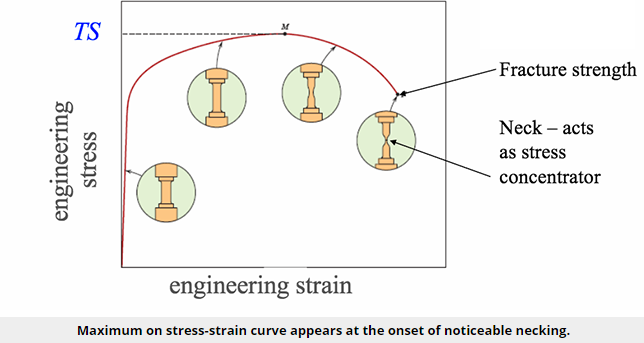
For many materials, the stress-strain curve looks like the curve shown in the figure below. As the stress is increased from zero, the strain increases linearly until it starts to deviate from linear at the yield strength. For increasing stress, the curve proceeds to a maximum at which point it curves downward toward the fracture point. The maximum corresponds to the tensile strength, which is the maximum stress value for the curve and is indicated by M in the figure. The fracture point is the point at which the material ultimately breaks, indicated by F in the figure.
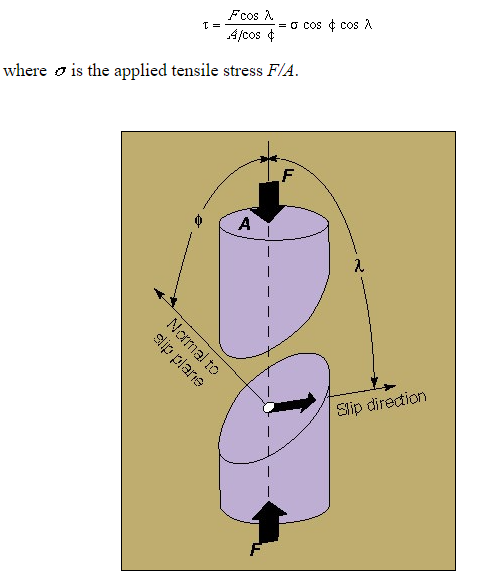
Crystalline slip results from the action of a shear stress on the slip plane. Within the range of stresses in natural situations, the component of stress normal to the slip plane does not influence slip. Thus, the slip process must be considered in terms of the shear stress resolved on the slip plane in the slip direction. Consider a single crystal of cross-sectional area A under a tensile force F (Fig. 2.10.1). Let 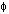 be the angle between the slip plane normal and the compression axis, and
be the angle between the slip plane normal and the compression axis, and 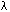 the angle between the slip direction and the tensile axis. The component of the applied force, acting in the slip direction is
the angle between the slip direction and the tensile axis. The component of the applied force, acting in the slip direction is 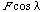 , and the area of the slip plane is
, and the area of the slip plane is 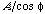 . The shear stress resolved in the slip direction is then
. The shear stress resolved in the slip direction is then
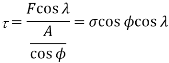
Where 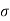 is the applied tensile stress
is the applied tensile stress 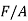
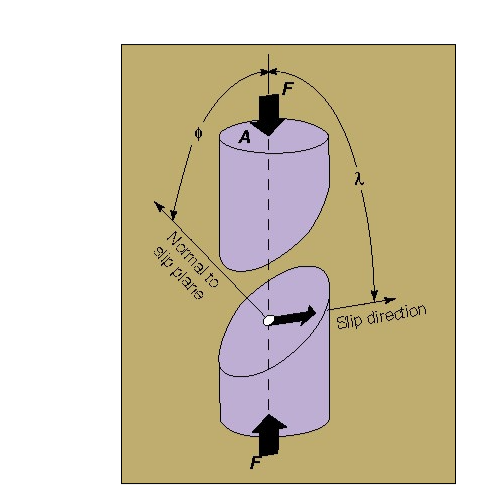
Two separated portions of a crystal showing a model for calculating the resolved shear stress in a single-crystal specimen. F is the applied force, A is the cross-sectional area of the specimen, 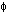 is the angle between the normal-to-the-slip plane and the compression axis, and
is the angle between the normal-to-the-slip plane and the compression axis, and 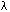 is the angle between the slip direction and the compression axis.
is the angle between the slip direction and the compression axis.
The stress required to initiate slip in a pure and perfect single crystal, the critical resolved shear stress (CRSS) is a constant for a material at a given temperature. This rule, known as Schmid's Law, has been experimentally proven for a large number of single crystals. The critical stress required to cause yielding is a function of 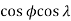 or the Schmid factor. The slip plane with the greatest resolved shear stress acting upon it will predominate in the slip process.
or the Schmid factor. The slip plane with the greatest resolved shear stress acting upon it will predominate in the slip process.
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to slide over each other at low stress levels and is known as glide or slip. The crystalline order is restored on either side of a glide dislocation but the atoms on one side have moved by one position. The crystalline order is not fully restored with a partial dislocation. A dislocation defines the boundary between slipped and unslipped regions of material and as a result, must either form a complete loop, intersect other dislocations or defects, or extend to the edges of the crystal.[1][2] A dislocation can be characterised by the distance and direction of movement it causes to atoms which is defined by the Burgers vector. Plastic deformation of a material occurs by the creation and movement of many dislocations. The number and arrangement of dislocations influences many of the properties of materials.
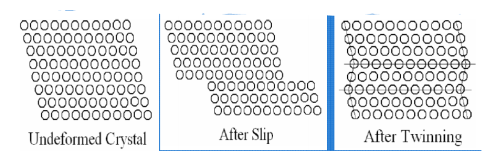
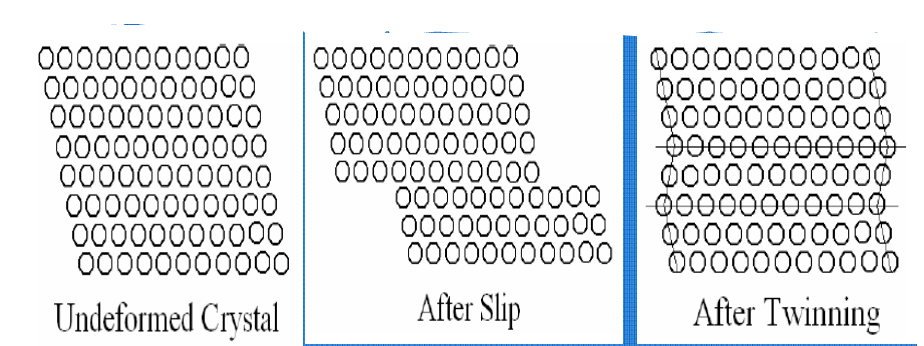
Two prominent mechanisms of plastic deformation, namely slip and twinning.
Slip
Twining:
Portion of crystal takes up an orientation that is related to the orientation of the rest of the untwined lattice in a definite, symmetrical way. The twinned portion of the crystal is a mirror image of the parent crystal. The plane of symmetry is called twinning plane. The important role of twinning in plastic deformation is that it causes changes in plane orientation so that further slip can occur.
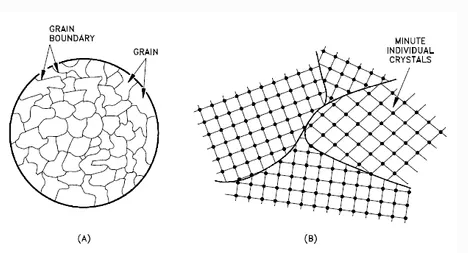
A crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances—that is, long-range order exists, such that upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest neighbor atoms. Not all solids are single crystals. For example, when liquid water starts freezing, the phase change begins with small ice crystals that grow until they fuse, forming a polycrystalline structure. In the final block of ice, each of the small crystals (called “grains “) is a true crystal with a periodic arrangement of atoms, but the whole polycrystal does not have a periodic arrangement of atoms, because the periodic pattern is broken at the grain boundaries.
Polycrystalline Structure
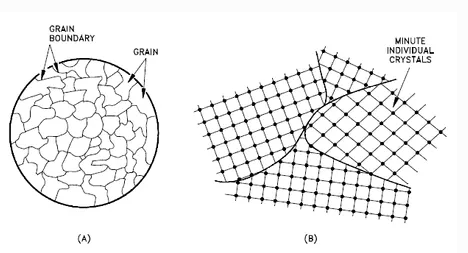
Not all solids are single crystals (e.g., silicon semiconductors). Most crystalline solids are composed of a collection of many small crystals or grains of varying size and orientation. These have random crystallographic orientations. When a metal starts with crystallization, the phase change begins with small crystals that grow until they fuse, forming a polycrystalline structure. In the final block of solid material, each of the small crystals (called “grains “) is a true crystal with a periodic arrangement of atoms, but the whole polycrystal does not have a periodic arrangement of atoms, because the periodic pattern is broken at the grain boundaries. Grains and grain boundaries help determine the properties of a material.
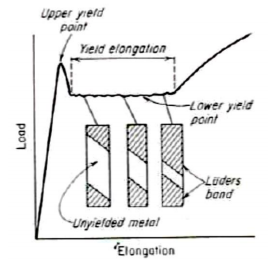
Metals, particularly low-carbon steel, show a localised heterogeneous transition from elastic to plastic deformation. →Yield point elongation
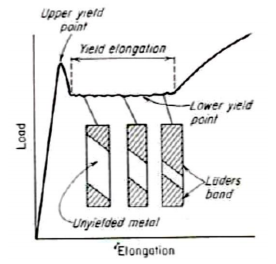
The load after the upper yield point suddenly drop to approximately constant value (lower yield point) and then rises with further strain. • The elongation which occurs at constant load is called the yield-point elongation, which are heterogeneous deformation. to the tensile axis o • Lüder bands or stretcher strains are formed at approximately 45 during yield point elongation and propagate over the specimen.
Cold working is deformation carried out under conditions where recovery processes are not effective. Hot working is deformation under conditions of temperature and strain rate such that recovery processes take place simultaneously with the deformation.
Structural changes during cold working of polycrystalline metals and alloys
1. Changes in shape and size of grains: The equiaxed grains on deformation are elongated in the direction of acting force i.e. stretched in the direction of main tensile deformation stress–say, in the direction of rolling or wire drawing.
2. Changes in orientation of grains: Preferred orientation or texture of is the state of severely cold worked metal in which certain crystallographic planes of the grains orient themselves in a preferred manner with respect to the direction of the stress (or maximum strain).
3. Changes in internal structure of grains: during cold working around 15% of the work of the deformation gets absorbed in the material (rest is lost as heat). This stored energy is the form of energy of crystal defects. Plastic deformation increases the concentration of point defects. With increase of cold working, the number of stacking-faults increases, thus density of extended dislocations increases. The number of kinks, jogs, dipoles, prismatic loops increase. The most important internal change of structure is increase in density of dislocation from 106 – 108 cm-2 in annealed state to 1010 – 1012 by moderate cold working.
Effect of cold work on properties
Cold working or strain hardening is the increase in the stress required to cause further slip because of previous plastic deformation. This is an important industrial process that is used to harden metals or alloys that do not respond to heat treatment. It changes various mechanical, physical and chemical properties of metals and alloys.
With increase in amount of cold work, Ultimate Tensile Strength, Yield Strength, Hardness increases but ductily (elongation and reduction in area) decreases. Cold worked texture and mechanical fibering leads to Anisotropy in in properties of materials. The ductility and impact toughness is much lower in transverse section rather than in longitudinal section. As the internal energy of cold worked state is high, the chemical reactivity of the material increases i.e. the corrosion resistance decreases, and may cause stress corrosion cracking in certain alloys. The rate of strain hardening (slope of flow curve) is generally lower in HCP metals than cubic metals. High temperatures of deformation also lower the rate of strain-hardening.
Annealing of Cold worked materials
In certain applications materials are used in the cold-worked state to derive benefits of increased hardness and strength. The cold worked dislocation cell structure is mechanically stable, but not thermodynamically stable. It is necessary to restore the ductility to allow further cold deformation or to restore the optimum physical properties such as electrical conductivity essential for applications. The treatment to restore the ductility or electrical conductivity with a simultaneous decrease in hardness and strength is Annealing (or Recrystallization annealing). It is heating cold worked metal to a temperature above recrystallization temperature, holding there for some time and then slow cooling.
The process of Annealing can be divided into three fairly distinct stages (1) Recovery (2) Recrystallization (3) Grain growth. There is no change in composition or crystal structure during annealing. The driving force for recovery and recrystallization is the stored cold-worked energy, whereas for grain growth is the energy stored in grain boundaries.
Recovery:
This usually occurs at low temperatures and involves motion and annihilation of point defects as well as annihilation and rearrangement of dislocations resulting in the formation of sub grains and sub grain boundaries (e.g., tilt and/or twist low-angle boundaries). A distinctive feature of the recovery process is that it does not involve any change in the grain structure of the cold-worked metal, the only changes taking place are the dislocation arrangements within the existing grains. Small changes in hardness that are sometimes observed during recovery can be attributed to the decrease in the dislocation and point defect density and to the growth of the sub grains.
Recrystallization:
If increased thermal activation is available (i.e., if the temperature is raised) nucleation and growth of strain-free grains in the deformed matrix will take place. As these grains grow, the dislocations in the matrix are annihilated at the boundaries of the newly formed grains. Strength and hardness decrease considerably and ductility increases. The lowest temperature at which stress-free grains appear in the structure of a previously plastically deformed metal is termed the recrystallization temperature. This depends upon the grain size, the severity of plastic deformation, and the presence of solute atoms or second phase particles. The recrystallization temperature is usually 1/3-1/2 the absolute melting point of the material.
Grain Growth:
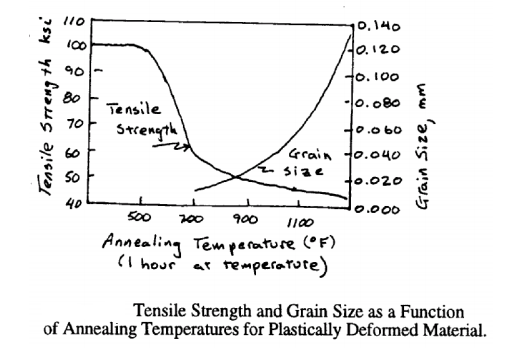
If a recrystallized material is further annealed at the same temperature or at a higher temperature grain growth usually occurs. Boundaries between annealed grains migrate and larger grains grow by an increase in the average grain size (or a decrease in the ASTM grain size number, n). Grain growth depends on the fact that the grain boundary energy of the material is reduced due to the decrease in grain boundary area for a given volume of material. The effect of recovery, recrystallization and grain growth on grain size, internal stress and strength (or hardness) of a plastically deformed material is illustrated schematically in Figure 5-
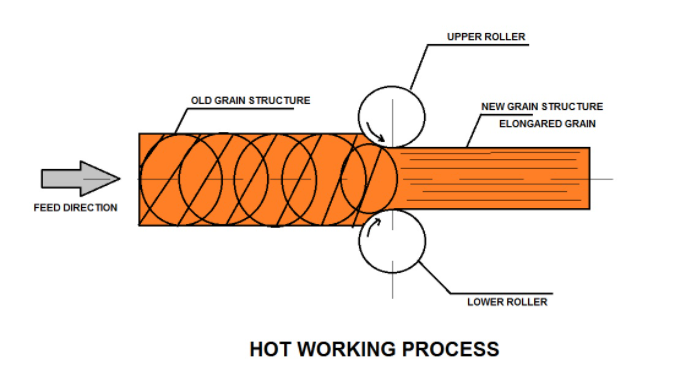
What is Hot-working Process?
Hot-working Process is a type of metal forming process and this distinction is based on the particular temperature at which the deformation is carried out. The minimum temperature at which plastically deformed metals form new grains or crystals within a specified time is recrystallization temperature.
In other words, we can say that when metal is deformed plastically above the recrystallization temperature but below the burning point it is called the hot working process of metal formation.
Method of Hot Working Process:
Various Methods of Hot Working Process are follows:
Hot Rolling
Hot Forging
Hot Extrusion
Hot Piercing or Rotary Piercing
Hot Drawing or Cupping and
Hot Spinning.
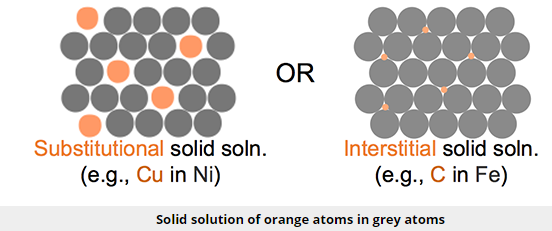
An alloy is a mixture of a metal with another element, either metal or nonmetal. If we start with a base metal and we add impurity atoms there are two possible outcomes if the two mix. The two different cases are highlighted in the figure below. In the substitutional solid case, the impurity atoms replace the host atoms in the lattice. In the interstitial situation, impurity atoms squeeze between the host atoms.
In addition to mixing, it is possible for regions of a new phase to form. An illustration of the formation of a second phase in a solid solution is shown below. The second phase can have a different composition and often a different structure.
Stainless Steel Alloys
Stainless steel is an alloy comprised of iron and carbon. You can differentiate between various steel alloys by the addition of other elements, such as nickel, copper, or manganese.
Stainless steel is famous for its use in kitchen appliances, cookware, and cutlery. Its stunning finishes range from matte to shiny, and it’s incredibly durable and easy to clean. Its anti-corrosive properties also make it useful in machinery applications.
Aluminium Alloys
On its own, aluminium isn’t the strongest metal—but when you add elements such as iron, copper, or zinc, you increase its strength and durability.
These alloys are lightweight and corrosion-resistant, and they’re often more affordable than other alloys. They also tolerate extreme temperatures very well. Aluminium alloys are common in mechanical engineering and aerospace manufacturing.
Bronze Alloys
Bronze contains copper and other additives. The additional ingredient may be tin, silicon, aluminium, manganese, phosphorus, or various other elements. Though they’re similar in color and appearance, bronze is not the same thing as brass—the latter is a combination of copper and zinc.
Bronze’s dull color is recognizable in medals, sculptures, and musical instruments. Its hardness and resistance to corrosion have also made it popular in ships and other nautical applications.
Nickel Alloys
Nickel is one of the most versatile metals. These alloys are often combinations of nickel and iron, copper, molybdenum, or chromium.
These alloys resist corrosion and oxidation well, and the wide range of compositions makes them applicable to several industries. This includes marine piping systems, pressure valves, or pump shafts. They’re also common in gas or steam turbines and medical equipment.
The applications of metal alloys are limitless. If you have any questions about the different types of metal alloys we offer, shop our selection or contact us to learn more.
Solid solution hardening is simply the act of dissolving one metal into another, similar to dissolving sugar into coffee. This is done during casting, when all the metals involved are in liquid form. For electrical connectors, copper is usually the main ingredient and is said to be the solvent, similar to the coffee in the above example. Other elements, playing the role of the sugar, to be added to the copper are known as the solutes.
There is a limit to the amount of solute that can be dissolved in to the solvent. This is known as the solubility limit. For example, coffee will only dissolve so much sugar before the excess settles on the bottom. However, raising the temperature of the solvent can often increase the solubility limit. There are several thermal strengthening methods that depend on having excess solute cast into the material and frozen into place when the mixture cools.
Plastic deformation (permanent set) of contact materials comes from the movement of dislocations throughout crystalline lattices that comprise each grain. Figure 1 shows how dislocations can move easily throughout an unalloyed metal. When other elements are dissolved into the copper, they help to impede the movement of dislocations. This imparts extra strength to the material.
The second type of solid solution is called an interstitial solution, such as carbon in steel. In this case, solute atoms are small enough to fit into spaces (interstices) between the solvent atoms in the crystal lattice (see Figure). Once again, the alloying element catches the dislocation and prevents it from moving further. It then requires greater stress or thermal energy for the dislocation to move around the impeding atom.
Introduction
A solution is a homogeneous mixture of one or more solutes in a solvent. Sugar cubes added to a cup of tea or coffee is a common example of a solution. The property which helps sugar molecules to dissolve is known as solubility. Hence, the term solubility can be defined as a property of a substance (solute) to dissolve in a given solvent. A solute is any substance which can be either solid or liquid or gas dissolved in a solvent. On this basis, the factors affecting solubility vary on the state of the solute:
Liquids In Liquids
Solids In Liquids
Gases In Liquids
Liquids In Liquids
Water is known as a universal solvent as it dissolves almost every solute except for a few. Certain factors can influence the solubility of a substance.
Solubility is the new bond formation between the solute molecules and solvent molecules. In terms of quantity, solubility is the maximum concentration of solute that dissolves in a known concentration of solvent at a given temperature. Based on the concentration of solute dissolves in a solvent, solutes are categorized into highly soluble, sparingly soluble or insoluble. If a concentration of 0.1 g or more of a solute can be dissolved in a 100ml solvent, it is said to be soluble. While a concentration below 0.1 g is dissolved in the solvent it is said to be sparingly soluble. Thus, it is said that solubility is a quantitative expression and expressed by the unit gram/ litre (g/L).
Based on solubility, different types of solution can be obtained. A saturated solution is a solution where a given amount of solute is completely soluble in a solvent at given temperature. On the other hand, a supersaturated solution is those where solute starts to salting out or precipitate after a particular concentration is dissolved at the same temperature.
Factors Affecting Solubility:
The solubility of a substance depends on the physical and chemical properties of that substance. In addition to this, there are few conditions which can manipulate it. Temperature, pressure and the type of bond and forces between the particles are few among them.
Temperature:By changing the temperature, we can increase the soluble property of a solute. Generally, water dissolves solutes at 20∘C or 100∘C. Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. But in case of gaseous substance, temperature inversely influences solubility i.e. as the temperature increases gases expand and escapes from their solvent.
Forces and Bonds:Like dissolves in like. The type of intermolecular forces and bonds vary among each molecule. The chances of solubility between two unlike substances are more challengeable than the like substances. For example, water is polar solvent where a polar solute like ethanol is easily soluble.
Pressure:
Gaseous substances are much influenced than solids and liquids by pressure. When the partial pressure of gas increases, the chance of its solubility is also increased. A soda bottle is an example of where CO2 is bottled under high pressure.
Solids In Liquids
It has been observed that solid solubility depends on the nature of solute as well as the solvent. We often see those substances like sugar, common salt (NaCl), etc readily dissolve in water while substances like naphthalene do not dissolve in water. From the various observations and experimental results, it has been seen that only polar solutes tend to dissolve in the polar solvent and non-polar solvents dissolve only non-polar solutes. Hence, nature of solvent can be seen as one of the prominent factors affecting solubility. The above observation led to the statement that like dissolves like, that is polar solvents will dissolve polar solutes and non-polar solvents dissolve non-polar solutes.
Crystallization: Now let us understand the process by which a solid dissolve in a solvent. Once a solid solute is added to a solvent, the solute particles dissolve in the solvent and this process is known as dissolution. Solute particles in the solution collide with each other and some of these particles get separated out of the solution, this process is called crystallization.
A state of dynamic equilibrium is established between these two processes and at this point, the number of solute molecules entering the solution becomes equal to the number of particles leaving the solution. As a result, the concentration of the solute in the solution will remain constant at given temperature and pressure.
A solution in which no more solute can dissolve in the solvent at a given temperature and pressure is said to be saturated solution as the solution contains the maximum amount of solute. The concentration of solute in such a solution is called its solubility at that temperature and pressure. If more solute can be added to a solution than it is called an unsaturated solution.
Factors Affecting Solubility
Effect of Temperature:Apart from the nature of solute and solvent, temperature also affects solid solubility considerably. If the dissolution process is endothermic than the solubility should increase with an increase in temperature in accordance with Le Chateliers Principle. If the dissolution process is exothermic the solid solubility should decrease.
Effect of Pressure:Solid solubility hardly gets affected by changes in pressure. This is due to the fact that solids and liquids are highly incompressible and practically do not get affected by changes in pressure.
Gases In Liquids
Gas solubility in liquids deals with the concept of gas dissolving in a solvent. Let us first define solubility. For any substance, solubility is the maximum amount of solute that can be dissolved in a given solvent at a particular temperature. Now our concern is gas solubility in liquids. The gas solubility in liquids is greatly affected by temperature and pressure as well as the nature of the solute and the solvent.
Size factor
This strengthening mechanism is based on the fact that crystallographic orientation changes abruptly in passing from one grain to the next across the grain boundary. Thus it is difficult for a dislocation moving on a common slip plane in one crystal to pass over to a similar slip plane in another grain, especially if the orientation is very misaligned. In addition, the crystals are separated by a thin non-crystalline region, which is the characteristic structure of a large angle grain boundary. Atomic disorder at the boundary causes discontinuity in slip planes. Hence dislocations are stopped by a grain boundary and pile up against it. The smaller the grain size, the more frequent is the pile up of dislocations. A twin boundary can also act as an obstacle to dislocation motion. A grain boundary can hinder the dislocation motion in two ways: (1) by forcing the dislocation to change its direction of motion and (2) discontinuity of slip plane because of disorder. Effectiveness of grain boundary depends on its characteristic misalignment, represented by an angle. The ordinary high-angle grain boundary (misalignment > 5) represents a region of random misfit between the grains on each side of the boundary. This structure contains grain-boundary dislocations which are immobile. However they group together within the boundary to form a step or grain boundary ledge. These ledges can act as effective sources of dislocations as the stress at end of slip plane may trigger new dislocations in adjacent grains. Small angle grain boundaries (misalignment < 1) are considered to be composed of a regular array of dislocations, and are not effective in blocking dislocations
Define Valency
The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element.
We all know how electrons in an atom are arranged in shells/orbitals. Valence electrons are those electrons which are present in the outermost orbit of the atom. From the Bohr-bury scheme, we can say that the outermost shell can contain a maximum of 8 electrons. Only a little chemical activity is observed when the outermost shell is completely filled. We can also say that its combining capacity becomes zero.
For example, nitrogen forms a number of compounds with hydrogen such as NH3, N2H4, N3H in which nitrogen atoms have valencies of 3, 2 and 1/3 respectively. Thus, this concept of valency as a mere number was not clear. Therefore, later on valency was defined as the number of chemical bonds formed by an atom in a molecule.
Concept of Valency
Noble gases have a completely filled outermost shell and that’s why they are least reactive. Other element’s reactivity depends upon their ability to attain the noble gas configuration. In this section, we shall learn more about the valency of an atom.
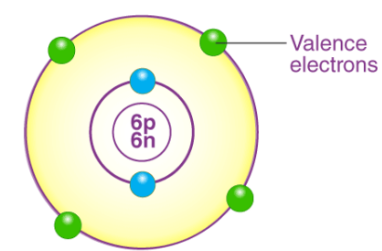
If the outermost shell has 8 electrons, then the element is said to have a complete octet. By gaining, sharing and losing the electrons the atoms complete their outermost orbital and make an octet.
The capacity of an atom is described by the total number of electrons lost, gained or shared to complete its octet and it also determines the valency of the atom.
Every atom in the unit cell contributes to every reflection according to its chemical nature and its relative position. Owing to this shift in position relative to the other atoms, the photons contributed by each atom in the unit cell have a phase shift relative to those from other atoms.
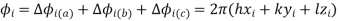
This makes the structure factor a complex number
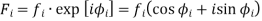
Every atom I in the unit cell contributes to every structure factor F(hkl) (that is reflection) according to its position in the cell and its chemical nature (different values for 
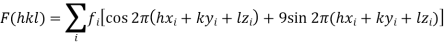
In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom or compound to combine by chemical reaction with atoms or compounds of unlike composition.
Definition
Order-disorder and order-order transitions refer to the phase transitions between the different phases of block copolymers. Block copolymers are macromolecules composed of chemically distinct sub-chains or blocks, which are capable of forming one disordered (homogeneous) phase and many ordered phases with nanodomain structures. The phase transitions between one of the ordered phases to the disordered phase are the order-disorder transitions (ODT), whereas the transitions between two ordered phases are termed order-order transitions (OOTs).
In physics, the terms order and disorder designate the presence or absence of some symmetry or correlation in a many-particle system.
In condensed matter physics, systems typically are ordered at low temperatures; upon heating, they undergo one or several phase transitions into less ordered states. Examples for such an order-disorder transition are:
the melting of ice: solid-liquid transition, loss of crystalline order;
the demagnetization of iron by heating above the Curie temperature: ferromagnetic-paramagnetic transition, loss of magnetic order.
The degree of freedom that is ordered or disordered can be translational (crystalline ordering), rotational (ferroelectric ordering), or a spin state (magnetic ordering).
The order can consist either in a full crystalline space group symmetry, or in a correlation. Depending on how the correlations decay with distance, one speaks of long-range order or short-range order.
If a disordered state is not in thermodynamic equilibrium, one speaks of quenched disorder. For instance, a glass is obtained by quenching (supercooling) a liquid. By extension, other quenched states are called spin glass, orientational glass. In some contexts, the opposite of quenched disorder is annealed disorder.
References:
1. Materials Science and Engineering by V.Raghavan, Prentice Hall of India Pvt.Ltd.
2. Elements of Materials Science & Engineering by Van Vlack, Pearson
3. Mechanical Metallurgy by Dieter, Tata MacGraw Hill
4. Composite Material science and Engineering by K. K. Chawla, Springer
5. Material Science and Metallurgy, by U. C. Jindal, Pearson