Unit – 1
Introduction
1.1.1 Composite Materials
A composite material is made up of two components that have distinct physical and chemical properties. When they're combined, they make a material that's tailored to a specific job, such as making it stronger, lighter, or more resistant to electricity, while also improving strength and stiffness.
Although the components function in unison, they do not disintegrate or entirely melt into one another within the composite.
We will study about composite materials, types of composite materials, and much more in this section.
1.1.2 Introduction to Composites Material:
Due to their versatility to a wide range of conditions, composite materials are possibly the most widely utilized materials.
It is simple to blend with various materials and has the efficiency of desirable qualities to perform certain functions.
The composite materials have a higher strength and hardness than bulk materials while having a lower density.
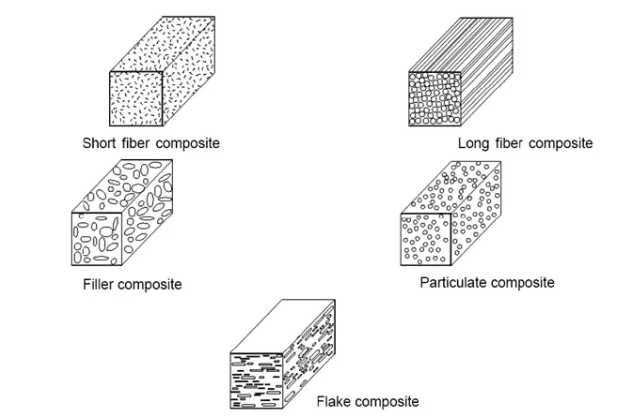
1.1.3 Classification of Composite Materials:
Microspheres:
They're thought to be among the most useful filters.
The most extreme property for switching products without losing profitability or physical qualities is gravity, fixed particle amplitude, power, and managed density.
Stable microspheres have a low density, which affects the completed product's commercial value and weight.
Their specific strength is covered in the finished mould in which they form a component, according to studies.
Hollow microspheres are silicate-based structures with a controlled specific gravity.
They're bigger than the stationary glass spheres used in polymers, and they come in a variety of particle sizes.
Filled composites:
The filler might be a primary component or a secondary component in a composite.
Filler particles can be random in shape or contain precise geometric shapes like polyhedrons, tiny fibers, or spheres.
Filled composer fillings can be used as a stand-alone component or as part of a composite.
Filler particles can be random in shape or contain precise geometric shapes like polyhedrons, tiny fibers, or spheres.
Flakes Structures:
Flakes are frequently substituted for fiber because they may be kept in dense packs.
Metal flakes in polymer matrixes that are in close contact with each other can conduct electrical energy or heat while opposing mica flakes and glass.
Flexes will be inexpensive to create and will typically cost less than fiber.
Particulate Reinforced Composites:
Particle reinforced composites are metal and ceramic composites with microstructures that exhibit particles of one phase distributed throughout another.
The square, triangular, and spherical reinforcement shapes are all recognized, but the proportions of all their sides are generally similar.
It differs from dispersion hardened material due to its size and volume concentration.
Laminar Composite:
Laminar composites can be regarded as materials in which layers of material are bonded together. They can be found as a number of materials in a variety of combinations.
As many layers of two or more metal components alternately or in a predefined order are required for a certain purpose, these can be made.
Fiber Reinforced Component:
Fibers are necessary for reinforcement because they meet specific requirements and impart strength to the matrix element, improving its qualities.
Key takeaway:
- A composite material is composed of at least two materials, which combine to give properties superior to those of the individual constituents.
- There are many types of composite materials such as carbon-reinforced fiber plastic, glass fiber–reinforced aluminum, composites with carbon nanotubes, and many more. Other types of composites include metal-matrix and ceramic-matrix composites. Composites have vast usage in engineering applications.
A composite is a material made by fusing two or more materials together to produce better qualities. Almost majority of the materials we perceive in our environment are composites. Woods, bones, and stones, for example, are natural composites since they are either formed in nature or formed through natural processes. Wood is a fibrous material made up of thread-like hollow elongated organic cellulose that makes up around 60-70 percent of wood, with 30-40 percent of it being crystalline and insoluble in water and the remainder being amorphous and soluble in water. Cellulose fibers have a high strength-to-weight ratio. The cellulose that is packed closer together has a higher density and strength. The principal load-bearing components of trees and plants are the walls of these hollow elongated cells. When trees and plants are alive, the load acting on a specific piece (for example, a branch) effects the growth of cellulose in the cell walls present there, reinforcing that part of the branch that is subjected to larger stresses. This self-strengthening mechanism is a one-of-a-kind phenomenon that may also be seen in living bones. Collagen fibers, which are inorganic calcium carbonate threads distributed in a mineral matrix called apatite, are found in bones. The fibers normally develop and become orientated in the load direction. Skeletons are the basic structural frameworks that sustain many sorts of static and dynamic loads in humans and animals. A tooth is a form of bone that has a flexible core and a hard enamel top. The tooth's compressive strength varies depending on its thickness. The exterior enamel is the most durable, having a compressive strength of up to 700 MPa. The tooth appears to contain piezoelectric capabilities, meaning that pressure causes reinforcing cells to develop. The low density, strong, and stiff fibers found in woods and bones are placed in a low-density matrix, resulting in a strong, stiff, and lightweight composite (Table 1.1). It's no surprise that wood was used as one of the primary structural materials in the early development of aeroplanes, and that about two hundred million years ago, huge flying amphibians, pterendons and pterosaurs, with wing spans of 8-15 m, could soar from the mountains like modern-day hang-gliders. In many ways, woods and bones might be regarded forerunners to modern man-made composites.
Early men effectively employed rocks, forests, and bones in their quest for survival against natural and external pressures. The primitive humans used these materials to manufacture weapons, tools, and a variety of utility items, as well as shelters. They mostly used these materials in their original state in the beginning. They gradually learned to make better use of them by cutting and moulding them into more useful shapes. They then used additional materials like as vegetable fibers, shells, clays, as well as animal horns, teeth, skins, and sinews.
Table 1.1 Typical mechanical properties of natural fibers and natural composites
Materials Density Tensile modulus Tensile strength |
Kg/m3 GPa MPa |
|
Fibers |
Cotton 1540 1.1 400 |
Flax 1550 1 780 |
Jute 850 35 600 |
Coir 1150 4 200 |
Pineapple leaf 1440 65 1200 |
Sisal 810 46 700 |
Banana 1350 15 650 |
Asbestos 3200 186 5860 |
Composites |
Bone 1870 28 140 |
Ivory 1850 17.5 220 |
Balsa 130 3.5 24 |
Spruce 470 11 90 |
Birch 650 16.5 137 |
Oak 690 13 90 |
Bamboo 900 20.6 193 |
The principal structural elements for creating shelters were woods, stones, and clays. As roofing materials, natural fibers such as grass plant straws and fibrous leaves were used. Stone axes, daggers, spears with wooden handles, wooden bows, fishing nets woven with vegetable fibers, jewelery and decorative pieces made of horns, teeth, semiprecious stones, minerals, and other materials were only a few instances of how people used those resources in the beginning. The drawbacks of employing these materials prompted a quest for better materials in order to create a more efficient material with superior qualities. This paved the way for the development of man-made composite materials.
The straw-reinforced clay that has sculpted society since prehistoric times is the most spectacular example of an early man-made composite. Egyptians were known to employ grass plant fibers to reinforce the clay-like deposits of the Nile Valley to manufacture sun-baked mud bricks that were used to build temple walls, tombs, and residences several hundred years B.C. During the Han Dynasty, the watchtowers on China's far western Great Wall were said to have been made with straw-reinforced bricks (about 200 years B.C.). Natural fiber reinforced clay is still one of the most common housing materials in many third-world countries' rural areas today.
The laminated wood furniture used by the early Egyptians (1500 B.C.) is another typical example, in which high-quality wood veneers are bonded to the surfaces of inferior woods. Paper created from plant fibers can be traced all the way back to China (108 A.D.). The Mongolian Chief Djingiz Chan's (1200 A.D.) warriors' bows were thought to be composed of an adhesive bonded laminated composite consisting of buffalo or anti-lope horns, wood, silk, and ox-neck tendons. These laminated composite bows could fire arrows with a range of around 740 metres.
Ceramic composites date back to the early days of pottery and hydraulic cement mortars. The ancient Chinese cloissone pottery is another stunning example of wire reinforced pottery. Fine copper wires were bent into appealing shapes before being covered in coloured clays and baked. Fine metallic wires of various sorts were cast using various metal and ceramic matrices in future years and used in a variety of applications. Natural gums and resins, rubbers, bitumen, shellac, and other matrix elements were also popular. Plant-based fibers (cotton, flux, hemp, etc.), animal-based fibers (wool, fur, and silk), and mineral-based fibers (asbestos) were in high demand. Royalty and the wealthy from all over the world patronized high-value textiles woven with beautiful gold and silver threads. During the Mughal dynasty in India, exquisite, artistic gold thread embroidery achieved its pinnacle. Glass fibers were first produced about 2000 years ago in Rome and Mesopotamia, and were widely employed in the decorating of flower vases and glass products of the time.
The emergence and development of a wide range of novel materials in the twentieth century has further solidified the foundation of modern composites. There have been numerous synthetic resins, metallic alloys, and ceramic matrices produced with superior physical, thermal, and mechanical qualities. Almost all materials have been used to pull fibers with a very small diameter (less than 10 m). They're a lot more durable and stiffer than the same material in bulk. When whiskers (single crystal fibers) are produced from some of these materials, the strength and stiffness qualities have been found to rise considerably. The specific tensile strength (M Pa) and modulus (G Pa) qualities are determined by dividing the strength (M Pa) and modulus (G Pa) by the density (kg m-3) or specific gravity of the material (see Figure 1.1). Owing to the
Because of the excellent mechanical qualities of fibers, their usage as reinforcements began to gain traction in the twentieth century. Fiber reinforced laminated plastics were first used to replace several metallic parts in the aircraft industry. Composite designers were captivated by fibers such as glass, carbon, boron, and Kevlar, as well as plastics such as phenolics, epoxies, and polyesters. Because of its greater specific modulus and strength qualities, utilizing fiber reinforced polymers (FRP) instead of metals almost always results in a weight-efficient design (Table 1.2).
Due to their heterogeneous makeup, composites offer an almost limitless number of ways to derive any desired material behaviour. This unique flexibility in design tailoring, as well as other benefits such as ease of manufacturing, particularly moulding to any shape with polymer composites, repairability, corrosion resistance, durability, adaptability, and cost effectiveness, have piqued the interest of a wide range of engineers and other professionals. Every industry is now competing to see who can employ composites the most effectively. Composites are presently used in a wide range of applications, from sports equipment to space transportation. This widespread interest has resulted in rapid growth in the field of composite materials and structures over the last four decades. A number of high-performance polymers have been developed in recent years. Stronger and stiffer fibers, metal and ceramic matrix composites, manufacturing and machining processes, quality control and nondestructive evaluation techniques, test methodologies, and design and analytic methodology have all made significant improvements. Modern man-made composites have thoroughly established themselves as the future material and are set to dominate the material landscape for the rest of the twenty-first century.
Table 1.2 Comparative mechanical properties of some man-made structural composites and metallic alloys
Materials Specific Tensile behavior Compressive Specific Specific Specific |
Gravity modulus strength strength tensile tensile compressive |
Modulus strength strength |
S E Xt Xc E/S Xt/S Xc/S |
G Pa M Pa M Pa G Pa M Pa M Pa |
Unidirectional Fiber Reinforced Plastics
GFRP 2.0 40 1650 1400 20.00 825.0 700.0 |
CFRP 1.6 140 1450 1050 87.50 906.3 656.3 |
KFRP 1.5 90 1650 300 60.00 1100.0 200.0 |
|
Metals |
Steel 7.8 206 400-2500 400-2500 26.40 50-320 50-320 |
Ti alloy 4.5 103 360-1400 360-1400 22.90 80-310 80-310 |
Al alloy 2.8 69 55-700 55-700 24.60 20-250 20-250 |
Mg alloy 1.8 47 150-300 150-300 25.00 83-166 83-166 |
Beryllium 1.8 303 400 400 168-33 222 222 |
1.3.1 Matrix Composites
Ceramics offer significant oxidation resistance and give strength at high temperatures well exceeding 15000C. They have a high elastic modulus, a high Peierls yield stress, low thermal expansion, poor thermal conductivity, a high melting point, superior chemical and weather resistance, and great electromagnetic transparency, among other properties. Ceramics, on the other hand, have a significant disadvantage in terms of plasticity. Ceramics' limited strain capability is a key source of concern, as it frequently leads to catastrophic failure. As a result, ceramics are not regarded as structurally sound materials. Ceramic matrix composites, on the other hand, may not have such limits, as appropriate reinforcements may help them attain desirable mechanical qualities, such as toughness. Glass, glass ceramics (lithium aluminosilicates), carbides (SiC), nitrides (SiN4, BN), oxides (Al2O3, Zr2O3, Cr2O3, Y2O3, CaO, ThO2), and borides are the most common ceramic matrices (ZrB2, TiB2). Particles, flakes, whiskers, and threads can be used as reinforcements, which are usually high-temperature inorganic materials such as ceramics. Carbon, silicon carbide, silica, and alumina are the most often utilized fibers. Ceramic matrix composites are experiencing a comeback in research and development due to their resistance to wear, creep, low and high cycle fatigue, corrosion, and impact, as well as their high specific strength at high temperatures. An alumina-SiC whisker cutting tool has a ten-fold higher cutting rate than traditional tools. Because of its high specific strength at high temperatures, ceramic composites can be used in aero-engine and automobile engine components to reduce weight and hence improve engine performance with higher thrust to weight ratios. Because of its light weight, automotive engines have higher efficiency, better performance at high operating temperatures, and a longer life due to exceptional heat and wear resistance. Table 2.9 lists a number of high-temperature uses for ceramic matrix composites.
Carbon-carbon composites are the most common type of ceramic matrix composite that can resist temperatures of up to 30000 degrees Celsius. They are made up of carbon fibers that are arranged in a carbon matrix. Pyrolysis of polymer impregnated carbon fiber fabrics and preforms under pressure or chemical vapour deposition of carbon or graphite are used to create them. The thermosets (furfurals, phenolics), thermoplastic pitches (coal tar and petroleum based), and carbon-rich vapours are the three types of polymers employed (hydrocarbons such as methane, propane, acetylene, benzene). In the production of carbon-carbon composites, phenolic resins are more typically used. On pyrolysis, the phenolic resin impregnated carbon fiber preforms transform to a significant fraction of amorphous carbon char. After the initial pyrolysis, the composite material is discovered to be porous. It is then impregnated with phenolic resin and pyrolised, usually under vacuum and pressure, with the process being repeated numerous times to reduce void content and achieve the material's ideal density. The main advantage of carbon-carbon composite is that different fabrics and shapes of preforms with multidirectional fiber alignments can be impregnated with resins and pyrolised to produce a wide range of one directional (1D), two directional (2D), three directional (3D), and multidirectional composite blocks of various shapes and sizes, which can then be machined to produce the desired dimensions. They are useful materials in high temperature applications because of their excellent wear resistance, increased coefficient of friction as temperature rises, high thermal conductivity, low thermal expansivity, and high temperature resistance. Carbon-carbon composites can sustain extremely high temperatures (30000C or more) for extended periods of time in the absence of oxygen. Because of their high biocompatibility, they're also used in prostheses.
1.3.2 Reinforcements
Fibers make up the lion's share of the reinforcements utilized in structural composites. A fiber is defined as a material with a minimum 1/d ratio of 10:1, where 1 is the fiber’s length and d are its smallest lateral dimension. The lateral dimension d (in the case of a circular fiber, the diameter) is considered to be smaller than 254 m. Fiber diameters used in structural composites typically range from 5 to 140 metres. A filament is a continuous fiber with an infinity l/d ratio. A whisker is a single crystal with a fiber-like appearance.
Lighter materials, particularly those based on low-atomic-number elements, are used to make common low-density fibers (e.g., H, Be, B, C, N, O, Al, Si, etc.). A fiber’s cross-section can be circular, as in glass, boron, and Kevlar fibers, although certain fibers can have regular prismatic cross-sections (such as whiskers) or random cross-sections (e.g., PAN, rayon and special pitch-based carbon fibers). Anisotropy in the fiber may be introduced by the uneven cross-section. Figure 2.1 depicts the typical microstructural morphology of common fibers.
Fibers can be amorphous (glass), polycrystalline (carbon, boron, alumina, etc.) or single crystals in terms of microstructure (silicon carbide, alumina, beryllium and other whiskers). A fiber’s strength and stiffness qualities are much greater than those of the bulk material from which it is made. The majority of ordinary fibers are fragile by nature. Because the shape and magnitude of a fault that the bulk material may possess controls the tensile strength, it is significantly lower than the theoretical strength. Because a fiber’s diameter is so small, any flaws it may include must be less than the diameter of the fiber. The criticality of the fault is reduced as a result of the lower flaw size, and thus the tensile strength is improved. A conventional glass (bulk) may have a tensile strength of 100-200 MPa, but an S-glass fiber may have a tensile strength of 5000 MPa. A flawless glass fiber, on the other hand, has a tensile strength of 10350 MPa based on intermolecular forces. Furthermore, crystallite arrangement along the fiber direction aids in significantly enhancing the strength qualities. A whisker is a single crystal that, unlike polycrystalline fibers, is not prone to crystal flaws and has extremely high strength and stiffness qualities. A graphite whisker has tensile strength and modulus of up to 25000 MPa and 1050 GPa, respectively. When compared to commercial fibers, these figures are fairly considerable. A commercially available PAN-based T300 fiber has typical longitudinal tensile parameters of 2415 MPa (strength) and 220 GPa (tension) (modulus). Tables 2.1 and 2.2 list typical thermomechanical and thermal characteristics of common fibers, respectively.
Structural composites can be made with both inorganic and organic fibers. Glass, boron, carbon, silicon carbide, silica, alumina, and other inorganic fibers (including ceramic fibers) are the most widely utilized. The number of structural grade organic fibers is relatively small. The most widely used organic fiber is aramid. Another recent addition is Spectra 900, a high-strength polyethylene fiber with a low density and great impact resistance. Carbon fibers are sometimes lumped in with organic fibers, however they're more commonly thought of as ceramic (inorganic) fibers. Inorganic fibers are robust, rigid, thermally stable, and moisture resistant in general. They are fatigue resistant, but have a low energy absorption capacity. Organic fibers, on the other hand, are less expensive, lighter, and more flexible than synthetic fibers. They have a higher strength and are more impact resistant.
Table 2.1 Typical mechanical properties of selected fibers
Fiber Material | Density kg/m3 | Tensile strength MPa | Tensile modulus GPa | Diameter �m |
Glass | 2550 | 3450-5000 | 69-84 | 7-14 |
Boron | 2200-2700 | 2750-3600 | 400 | 50-200 |
Carbon | 1500-2000 | 2000-5600 | 180-500 | 6-8 |
Kevlar | 1390 | 2750-3000 | 80-130 | 10-12 |
Polyethylene | 970 | 2590 | 117 | 38 |
Silica (SiO2) | 2200 | 5800 | 72 | 35 |
Boron carbide (B4C) | 2350 | 2690 | 425 | 102 |
Boron nitride | 1910 | 1380 | 90 | 6.9 |
Silicon carbide (SiC) | 2800 | 4500 | 480 | 10-12 |
TiB2 | 4480 | 105 | 510 | - |
TiC | 4900 | 1540 | 450 | - |
Zirconium oxide | 4840 | 2070 | 345 | - |
Borsic (SiC/B/W) | 2770 | 2930 | 470 | 107-145 |
Alumina (Al2O3) | 3150 | 2070 | 210 | 17 |
Alumina FP | 3710 | 1380 | 345 | 15-25 |
Steel | 7800 | 4140 | 210 | 127 |
Tungsten | 19300 | 3170 | 390 | 361 |
Beryllium | 1830 | 1300 | 240 | 127 |
Molybdenum | 1020 | 660 | 320 | 127 |
Quartz whisker | 2200 | 4135 | 76 | 9 |
Fe whisker | 7800 | 13,800 | 310 | 127 |
SiC whisker | 3200 | 21,000 | 840 | 0.5-10 |
Al2O3 whisker | 4000 | 20,700 | 427 | 0.5-10 |
BeO whisker | 2851 | 13,100 | 345 | 10-30 |
Fiber Material | Density kg/m3 | Tensile strength MPa | Tensile modulus GPa | Diameter �m |
B4C whisker | 2519 | 13,790 | 483 | - |
Si3N4 whisker | 3183 | 13,790 | 379 | 1-10 |
Graphite whisker | 2100 | 20,800 | 1000 | - |
Table 2.2: Typical thermal properties of selected fibers
Fiber | Melting point 0C | Heat Capacity KJ/(kg.K) | Thermal Conductivity W/(mK) | Coefficient of thermal expansion 10-6 m/mK |
Glass | 840 | 0.71 | 13 | 5 |
Boron | 2000 | 1.30 | 38 | 5 |
Carbon | 3650 | 0.92 | 1003 | -1.0 |
Kelvar 49 | 250 | 1.05 | 2.94 | -4.0 |
SiC | 2690 | 1.2 | 16 | 4.3 |
Steel | 1575 | 0.5 | 29 | 13.3 |
Tungsten | 3400 | 0.1 | 168 | 4.5 |
Beryllium | 1280 | 1.9 | 150 | 11.5 |
Molybdenum | 2620 | 0.3 | 145 | 4.9 |
Fe whisker | 1540 | 0.5 | 29 | 13.3 |
Al2O3 whisker | 2040 | 0.6 | 24 | 7.7 |
Quartz Whisker | 1650 | 0.963 | 10 | 0.54 |
Glass, silica, quartz, and carbon fibers, as well as organic fibers, are commercially available as strands, tows, or yarns. A filament strand (or end) is a grouping of filaments. A tow (or roving) is made up of numerous strands or ends. A twisted strand is referred to as a yarn. For compactness and to make a composite with a higher fiber content, some twist is preferred. Excessive twisting, on the other hand, should be avoided because the matrix may not be able to enter and moisten all of the fibers. Woven rovings and woven fabrics are also made from these fibers (clothes). Fabric weave styles include unidirectional (uniaxial), bidirectional (biaxial 2D and biaxial 3D), and multidirectional (biaxial 2D and biaxial 3D) (multiaxial). Wrap fibers are yarns that are put along the roll in a uniaxial fabric wrap. Fill/pick fibers are put in the weft direction, which is transverse to the roll direction, and make up a minor portion of the wrap fibers. In most situations, the weaving design process and weaving processes follow comparable approaches to those used in textile technology. Figures 2.2 and 2.3 depict a few common weave patterns. Fabrics for carbon-carbon composites may use sophisticated multidirectional weaving patterns, whereas commercial woven rovings use a simple plain weave technique. Hybrid fabrics can also be made by weaving different fibers together in the warp and weft orientations. Preimpregnated woven rovings and textiles are frequently used to make prepregs that can be used in the manufacturing of composite parts.
1.4.1 Factors That Determine the Mechanical Properties of a Metal
Many aspects must be addressed while selecting materials for engineering uses. Manufacturers are aware that each metal alloy has its own set of qualities that react differently to mechanical and chemical processes. Understanding these qualities and determining which alloy is best suited for the work at hand is critical in order to maximize the efficiency and cost savings of any job.
Grain size, heat treatment, air exposure, and temperature are all important elements in determining the mechanical characteristics of metal. These characteristics work together to influence how a metal reacts to the stresses it encounters in industrial operations. Manufacturers must thoroughly test alloys to determine how they will be affected and under what conditions they will fail.
Different procedures have different effects on metals. The concept of stress and strain is an important one. When comparing specimens of different sizes, the load per unit area, also known as normalization to the area, must first be calculated. The force is divided by the area to calculate stress. The relevant area while conducting tension and compression testing is perpendicular to the force. For shear or torsion testing, on the other hand, the relevant region is perpendicular to the rotation axis.
Metal alloys can react in unfavorable ways as a result of stress and strain, so they must be thoroughly examined. The term "elastic deformation" refers to a condition in which the material can return to its original dimensions if the stress is eliminated. The ability to remain stable under stress, as well as the fact that the deformation is reversible and non-permanent, is indicated by the elasticity. Plastic deformation, on the other hand, indicates that the metal is unable to return to its original shape after the stress is removed. Instead, the tension has resulted in irreversible permanent deformity.
Metal's mechanical characteristics are influenced by a variety of factors. Changes in grain size, for example, affect yield strength, hardness, the ductile-brittle transition temperature, and susceptibility to environmental conditions, all of which can be improved.
Metals, such as aluminium, are made up of crystals, which are also called grains. Fine-grained aluminium is defined as having a tiny grain size, whereas coarse-grained aluminium has a big grain size. Fine grain aluminium alloys have higher tensile and fatigue strength than coarse grain aluminium alloys. Work hardening is easier with these alloys. Aluminum with coarser granules has a rougher surface and is harder to polish. The fact that coarse-grained aluminium is less robust and thus more likely to suffer irreversible distortion under stress is another effect of grain. Coarse-grained aluminium, on the other hand, provides advantages in terms of workability, hardenability, and forgeability. They also have distinct reactions at different temperatures. Fine-grained aluminium is stronger and tougher at room temperature, whereas coarse-grained aluminium has greater creep resistance at higher temperatures. The link can be determined using a simple formula: the strength of a metal is inversely related to the square root of the grain size. Temperature has an impact on a metal's mechanical properties, such as its tenacity and elastic limit. Heat treatment improves the mechanical qualities of aluminium and other metals, such as ductility, hardness, tensile strength, toughness, and shock resistance, and is used in many industrial operations. When it comes to aluminium alloys, heat treatment has various advantages. It has the ability to refine grain and increase machinability. Working with metal, whether hot or cold, causes internal tension, and heat treatment is one technique to relieve that tension. A changed grain structure and increased corrosion resistance, as well as more favorable chemical, magnetic, electrical, and thermal characteristics, are all added benefits. Atmospheric corrosion is a serious concern for metals, and it must be taken into account by producers. When metals are exposed to the atmosphere for long periods of time, they oxidize. This oxidation of the metal surface forms a coating, which is more common in the presence of moisture, sulphur dioxide, and hydrogen sulphide, and lowers the material's electrical resistivity. The atmospheric effect is influenced by a number of elements, including the metal's properties, the protective surface film's quality, the presence of particular chemicals that might lessen corrosive effects, and the presence of surface fissures or discontinuities. Once again, knowing what environmental conditions the aluminium alloy will be exposed to during the length of its product life is critical. Low and high temperatures have varying effects on aluminium alloys, depending on their mechanical qualities. In general, when the temperature drops, the tensile and yield strength of the material increases. At low temperatures, aluminium alloys, as well as nickel and copper, retain the majority of their hardness and ductility. Non-ferrous metals retain their characteristics better than ferrous metals at extremely low temperatures of less than -100 degrees Celsius. Modest temperatures can also cause low thermal vibrations and lattice properties to stabilise. Field stress and tensile strength, as well as stiffness and fracture stress, decrease as the temperature rises at high temperatures. Steel is particularly vulnerable to high temperatures, and its hardness suffers as a result. This is due to the fact that when the temperature rises, the thermal vibration of atoms increases, causing changes in the metal's structural properties. In the meantime, many aluminium alloys will maintain their mechanical qualities at high temperatures. Aluminum offers a variety of mechanical qualities that make it ideal for industrial applications. In comparison to steel, copper, and brass, it is lightweight and has high corrosion resistance in a variety of environments. It has a high reflectivity, which makes it ideal for aesthetic applications. Despite their lower density, several aluminium alloys can actually outperform steel in terms of strength. It maintains its hardness at low temperatures and is a good heat and electrical conductor, making it an excellent choice for electronic applications. Aluminum conductivity is 204 percent that of copper when weighted equally. It's easy to polish, and it's also more machinable. It's also non-toxic, so it can be employed in a variety of food and beverage applications. Aside from that, aluminium is very simple to recycle. Aluminum alloys are a high-quality, durable, and versatile material for your production needs. Clinton Aluminum believes in “The Right Alloy for the Right Application,” and we are proud to be a technical resource partner to our suppliers and customers.
Benefits of Composites
Almost any article which can be produced in traditional materials such as metals can be manufactured from composites. Whilst the use of composites will be a clear choice in many instances, material selection in others will depend on factors such as working lifetime requirements, number of items to be produced (run length), complexity of product shape, possible savings in assembly costs and on the experience and skills of the designer in tapping into the optimum potential of composites. In some instances, best results may be achieved through the use of composites in conjunction with traditional materials. Let’s have a brief look at the benefits of composites.
Light Weight
Composites are light in weight compared to most woods and metals. Their lightness is important in automobiles and aircraft, for example, where less weight means better fuel efficiency. Designers of airplanes are greatly concerned with weight, since reducing a craft’s weight reduces the amount of fuel it needs, and increases the speeds it can reach. Some modern airplanes are built with more composites than metal – including the Boeing 787 Dreamliner.
High Strength
Composites can be designed to be far stronger than aluminium or steel. Metals are equally strong in all directions. But composites can be engineered and designed to be strong in a specific direction.
Strength Related to Weight
Strength-to-weight ratio is a material’s strength in relation to how much it weighs. Some materials are very strong and heavy, such as steel. Other materials can be strong and light, such as bamboo poles. Composite materials can be designed to be both strong and light. This property is why composites are used to build airplanes—which need a very high strength material at the lowest possible weight. A composite can also be made to resist bending in one direction, for example. When something is built with metal, and greater strength is needed in one direction, the material usually must be made thicker, which adds weight. Composites can be strong without being heavy. Composites have the highest strength-to-weight ratios in structures today.
Corrosion Resistance
Composites can withstand the elements as well as severe chemicals that can eat away at other materials. Where chemicals are handled or stored, composites are a viable alternative. Outside, they can withstand extreme weather and temperature swings.
High-Impact Strength
Composites can withstand the elements as well as severe chemicals that can eat away at other materials. Where chemicals are handled or stored, composites are a viable alternative. Outside, they can withstand extreme weather and temperature swings.
Design Flexibility
Composites are easier to mould into complex shapes than most other materials. This allows designers to build practically any shape or form they choose. Fiberglass composites, for example, are used in the construction of most recreational boats today because they can be easily moulded into complicated shapes, improving boat design while cutting prices. Composites' surfaces can be moulded to resemble any surface finish or texture, from smooth to pebbly.
Part Consolidation
A single piece made of composite materials can replace an entire assembly of metal parts. Reducing the number of parts in a machine or a structure saves time and cuts down on the maintenance needed over the life of the item.
Dimensional Stability
When composites are hot or cold, wet or dry, they keep their shape and size. Wood, on the other hand, swells and shrinks in response to changes in humidity. In circumstances where tight, consistent fits are required, composites may be a preferable option. They're employed in aircraft wings, for example, to keep the shape and size of the wings consistent as the plane gains or loses altitude.
Nonconductive
Nonconductive means that composites do not conduct electricity. This feature qualifies them for applications like as electrical utility poles and electronic circuit boards. Some composites can be made conductive if electrical conductivity is required.
Nonmagnetic
Because composites do not include any metals, they are not magnetic. They can be used around electronic equipment that is sensitive. Large magnets used in MRI (magnetic resonance imaging) equipment function better because of the lack of magnetic interference. Both the equipment housing and the table are made of composites. In addition, composite rebar was used in the room's construction to reinforce the hospital's concrete walls and flooring.
Radar Transparent
Composites are great materials for use whenever radar equipment is used, whether on the ground or in the air, because radar signals pass directly through them. Composites are used extensively in stealth aircraft, notably as the US Air Force's B-2 stealth bomber, which is virtually radar-invisible.
Low Thermal Conductivity
Composites are good insulators—they do not easily conduct heat or cold. They are used in buildings for doors, panels, and windows where extra protection is needed from severe weather.
Durable
Composite structures have a long lifespan and require little maintenance. We don't know how long composites survive because many original composites haven't reached the end of their useful life. Many composites have been in use for over 50 years.
Reinforcements: An Overview Fibers, textiles particles, and whiskers can all be used as reinforcement in composites. Fibers are defined by one very long axis and two additional axes that are often circular or nearly circular. Particles, like their shapes, have no preferred orientation. Whiskers have a distinctive shape, however they are relatively modest in diameter and length when compared to fibers. The types of reinforcements used in composites are depicted in Figure M1.2.3.
1.6.1 Reinforcement
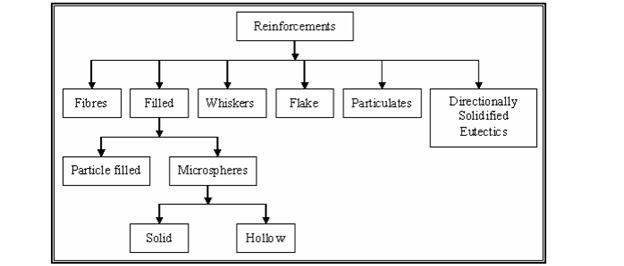
As the name implies, reinforcing elements in composites provide the strength that makes the composite what it is. However, they also fulfil the functions of heat resistance or conduction, corrosion resistance, and stiffness. According to the requirements, reinforcement can be created to fulfil all or any of these functions. A reinforcement that adds to the matrix's strength must be stronger and stiffer than the matrix, as well as capable of modifying the failure mechanism to the composite's benefit. This means that ductility should be low, if not non-existent, and the composite should be as brittle as feasible.
1.6.2 Reinforcement matrix interface
The matrix is a completely continuous monolithic material into which the reinforcement is embedded. Unlike two materials sandwiched together, there is a channel through the matrix to any location in the material. The matrix is commonly a lighter metal, such as aluminium, magnesium, or titanium, in structural applications, and offers a compliant support for the reinforcement. Cobalt and cobalt-nickel alloy matrices are used in high-temperature applications.
The matrix constituent is a typical way to classify composite materials. Organic Matrix Composites (OMCs), Metal Matrix Composites (MMCs), and Ceramic Matrix Composites are the three basic composite classifications (CMCs). The term "organic matrix composite" is widely used to refer to two types of composites: polymer matrix composites (PMCs) and carbon matrix composites, often known as carbon-carbon composites.
Three common forms of composites result from these three sorts of matrices.
1. Ceramic fibers are used in a plastic matrix in polymer matrix composites (PMCs), of which GRP is the most well-known example.
2. Silicon carbide fibers are commonly inserted in an alloy of aluminium and magnesium in metal-matrix composites (MMCs), but other matrix materials such as titanium, copper, and iron are increasingly being employed. Bicycles, golf clubs, and missile guidance systems are all examples of MMC applications; an MMC constructed of silicon carbide fibers in a titanium matrix is now being researched for use as the skin (fuselage material) of the US National Aerospace Plane.
3. Ceramic-matrix composites (CMCs) are the third major category, with silicon carbide fibers set in a borosilicate glass matrix as an example. They are particularly well suited for usage in lightweight, high-temperature components, such as parts for aeroplane jet engines, due to their ceramic matrix.
Several factors influence the selection of a matrix alloy for an MMC. It's crucial to know if the composite will be reinforced constantly or intermittently. When continuous fibers are used as reinforcements, the majority of the load is transferred to the reinforcing filaments, and composite strength is mostly determined by fiber strength. The matrix alloy's principal functions are to transport load efficiently to the fibers and to soften cracks in the case of fiber failure, hence the matrix alloy for continuously reinforced composites may be chosen for toughness rather than strength.
In continuously reinforced composites, lower strength, more ductile, and harder matrix alloys may be used. The matrix may control composite strength in discontinuously reinforced composites. The choice of matrix will then be determined by the necessary composite strength, which may necessitate the use of higher strength matrix alloys. Potential reinforcement/matrix reactions, either during processing or in service, which could result in degraded composite performance; thermal stresses due to thermal expansion mismatch between the reinforcements and the matrix; and the influence of matrix fatigue behaviour on the cyclic response of the composite are all factors to consider when choosing the matrix. Indeed, the behaviour of composites under cyclic loading situations is something that needs to be taken into account. The difference in melting temperatures between the matrix and the reinforcements is an additional issue in composites intended for use at high temperatures. Even at temperatures near the matrix melting point, a substantial melting temperature differential can cause matrix creep while the reinforcements remain elastic. When there is a tiny melting point difference in the composite, however, creep in both the matrix and reinforcement must be considered.
1.7.1 What are Natural Fibers?
The term "natural fiber" refers to fibers that are acquired from (or produced by) animals and plants. These fibers are used in the production of composite materials in a variety of ways. Matting different layers of natural fibers into sheets can be used to make paper and felt (a type of textile material).
Most natural fibers are well-known for their ability to absorb sweat and other liquids. Natural fibers can be used to create a wide range of textures (either individually or through a combination of two or more natural fibers). Cotton fibers (natural fibers generated from the cotton plant) are used in the creation of cotton garments that are known for their low weight and delicate texture. Cotton fiber also has the advantage of being able to be weaved into clothes of various sizes and colours. Clothing made of natural fibers (such as cotton) is frequently chosen over clothing made of synthetic fibers, particularly by those who live in hot and humid climates.
1.7.2 Examples of Natural Fibers
Plant fibers and animal fibers are the two primary groups of natural fibers. In this subsection, examples of plant and animal fibers have been offered.
Plant Fibers
• Seed fibers are the fibers derived from the seeds of many plants.
• Leaf fibers — natural fibers extracted from the leaves of specific plants. Pineapple and banana leaf fibers are two examples.
• Fruit fibers are the natural fibers obtained from a plant's fruit (coconut fiber, for example).
• Stalk fibers – these are natural fibers derived from the stalks of some plants. Wheat straws, bamboo fibers, fibers obtained from the stalks of rice and barley plants, and straw are examples.
• Bast fibers — natural fibers derived from the outer layer of the stem's cells. Jute fibers, flax fibers, vine fibers, industrial hemp fibers, kenaf fibers, rattan fibers, and ramie fibers are all examples of bast fibers. These fibers are commonly utilized in fabric and packaging due to their long-lasting properties.
Animal Fibers
Animal fibers are natural fibers that contain proteins such as fibroin, keratin, and collagen. The following are some common examples of animal fibers.
• Silk is a form of animal fiber derived from silkworms (different species produce different types of silk).
• Sinew — an animal fiber that joins the muscles and bones of certain animals.
• Wool is an animal fiber obtained by shearing the fur of specific sheep breeds.
• Mohair is an animal fiber made from the Angora goat's hair.
1.7.3 Applications of Natural Fibers
Natural fibers, including certain glass fibers, are widely employed in the construction industry in a variety of building materials. Even when put in a matrix of synthetic polymers, such composites (also known as bio composites) can be considered natural fibers. Cellulose fiber offers a wide range of applications in a variety of industries, including automotive and electronics. These natural fibers can be utilized for noise-absorbing panels and insulation.
Silk, wool, angora, and camel hair are the four most valuable animal fibers in terms of industrial value. Many plant fibers have important industrial applications as well. Cotton fiber, for example, is an important raw resource in the textile industry. Hemp fiber, jute fiber, and flax fiber are also important plant fibers in industry.
Natural fibers may also have medical applications since they can aid in the production of biomaterials. The natural fiber Chitin, for example, can be used to filter out certain hazardous contaminants from industrial wastewater.
Register with BYJU'S and download the mobile application on your smartphone to learn more about natural fibers and other related themes.
Key takeaways:
- Fibers derived from bio-based sources such as vegetables and animal origin are termed as natural fibers. This definition includes
All natural cellulosic fibers (cotton, jute, sisal, coir, flax, hemp, abaca, ramie, etc.) and protein-based fibers such as wool and silk.
- Natural fibers come from many sources. These sources can include plants, animals, and minerals. We are probably most familiar with plant and animal fibers from a consumer standpoint. Common natural fibers sourced from the plant kingdom include cotton, flax, hemp, bamboo, sisal, and jute.
1.8.1 What is Polyethylene?
Polyethylene, often known as polythene or polyethene, is a type of plastic that is widely used around the world. Polyethylene’s are known to be addition polymers and have a linear structure. Packaging is the most common use for these synthetic polymers. Plastic bags, bottles, plastic films, containers, and geomembranes are all made from polyethylene. It's worth noting that about 100 million tonnes of polyethene are manufactured each year for commercial and industrial use.
Polyethylene can be expressed as (C2H4)n in its generic formula. The majority of polyethylene is thermoplastic (they can be remoulded by heating). Some modified polyethylene polymers, on the other hand, have thermosetting qualities. Cross-linked polyethylene is an example of this type of polyethylene (often abbreviated to PEX).
Ethylene is the most important component of polyethylene (an organic hydrocarbon with the chemical formula C2H4; IUPAC name: ethene). The normal parameters for polyethylene manufacture include less than 5 parts per million of oxygen, water, and other alkenes. Other pollutants can, however, be present during the polymerization reaction. Nitrogen, methane, and ethane are among of the most widely recognized pollutants in polythene manufacture.
Because ethene is a rather stable chemical, it requires special catalysts to polymerize it. It's worth noting that the process of converting ethylene to polyethylene is highly exothermic. Titanium (III) chloride is one of the most regularly used catalysts for the polymerization of ethylene (which is sometimes referred to as a Ziegler-Natta catalyst).
1.8.2 Uses of Polyethylene
• The most common use of polyethylene is in packaging materials. Plastic bags, plastic films, bottles, geomembranes, and containers are frequently made from this material.
• Crates, trays, milk or fruit juice containers, and other food packaging products are made of polyethylene.
• Toys, garbage cans, ice trays, and other household items are made of high-density polyethylene. This plastic's versatility makes it suitable for a wide range of applications.
• Ropes, fishing nets, agricultural nets, and industrial fabrics are all made of HDPE. This material is also commonly used in wiring and cables.
• Because of its great flexibility and low cost, low-density polyethylene (LDPE) is frequently utilized in the production of squeeze bottles, waste bags, laminations, and food packaging
• LDPE is also utilized in the manufacture of pipes and fittings. Because of its minimal water absorption and flexibility, it is perfect for such applications.
• Because it is a good insulator of electric current, polyethylene is often utilized for cable jacketing.
1.8.3 Aramid
Any of a range of synthetic polymers (substances made up of long chainlike multiple-unit molecules) in which repeating units containing big phenyl rings are bonded together by amide groups (full aromatic polyamide). Amide groups (CO-NH) produce strong, solvent- and heat-resistant bonds. Phenyl rings (also known as aromatic rings) are six-sided carbon and hydrogen atom groups that keep polymer chains from rotating and twisting around their chemical connections. As a result, aramids are stiff, straight, high-melting, and mostly insoluble molecules, making them excellent for spinning into high-performance fibers. Nomex, a high-melting fiber used in flame-resistant protective equipment, and Kevlar, a high-strength fiber used in bulletproof vests, are the most well-known aramids.
Nylon, a related class of polyamides made by reacting acids having carboxyl groups (CO2H) with substances containing amino groups, was developed before aramids (NH2). Researchers at E.I. Du Pont de Nemours & Company (now DuPont Company) in the United States developed methods for extending this class to compounds with carbon rings in the 1950s and 1960s. These procedures entailed dissolving the acids and amines in suitable solvents and reacting them at low temperatures, as devised by Paul W. Morgan and Stephanie L. Kwolek. DuPont introduced Nomex, or poly-m-phenylene isophthalamide, in 1961, made from isophthalic acid chloride and m-phenylenediamine, and Kevlar, or poly-p-phenylene terephthalamide, in 1971, made from terephthalic acid chloride and p-phenylenediamine. The structure of the molecules distinguishes these two polymers, with Nomex having meta-oriented phenyl rings and Kevlar having para-oriented phenyl rings.
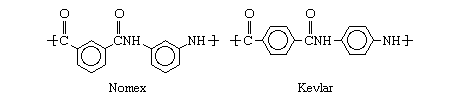
Nomex melts and decomposes at around 350 degrees Fahrenheit (660 degrees Fahrenheit); Kevlar melts at temperatures around 500 degrees Fahrenheit (930 degrees Fahrenheit). Kevlar's higher melting temperature, as well as its stiffness and tensile strength, are due in part to its molecules' regular para-orientation. The polymer takes on a liquid-crystal structure in solution, which allows the molecules to be spun and pulled into highly ordered fibers with ultrahigh stiffness and strength. (Kevlar is five times stronger than steel in terms of weight.) Twaron (from the Dutch business Akso NV) and Technora are two more trademarked Kevlar-type fibers (from the Japanese company Teijin, Ltd.). Teijin also produces a Nomex-like fiber under the Conex brand.
Although aramids are rarely produced in the same amount as commodity fibers like nylon and polyester, their high unit price makes them a lucrative market. End uses for aramids in the house are limited (Nomex-type fibers have been used to make ironing board covers), but industrial applications are growing (especially for aramids of the Kevlar class) as product designers learn how to take advantage of these strange materials' qualities. Kevlar and its competitors are used in radial tyre belts, cables, reinforced composites for aircraft panels and boat hulls, flame-resistant garments (especially in blends with Nomex), and sports equipment such as golf club shafts and lightweight bicycles, as well as bestos replacements in automobile clutches and brakes. Filters for hot-stack gases, clothes for presses applying permanent-press finishes to fabrics, dryer belts for papermakers, insulation paper and braid for electric motors, flame-resistant suits for fire fighters, military pilots, and race-car drivers, and automobile v-belts and hoses are all made from Nomex-type fibers.
1.9.1 Inorganic Fibers
Glass fiber, amorphous fiber like rock wool, carbon fiber, polycrystal fiber like alumina fiber, and monocrystal fiber like wollastonite and potassium titanate fiber are all examples of inorganic fibers. Because there is no grain boundary, amorphous fiber has a high strength despite having a low modulus elasticity. Because polycrystalline fiber is made up of tiny crystals, it has a higher heat resistance. Because of the whisker-like tiny fibers, monocrystalline fiber has an incredibly high strength.
Glass fiber
Chopped strands with a fiber diameter of 6–20 m and a fiber length of 3–25 mm with exceptional heat resistance and dimension stability are the glass fibers most commonly used for papermaking. Because of its properties, glass fiber sheets are utilized for flooring, insulation, and building materials.
Carbon fiber
Carbon fibers are divided into two categories: Types based on PAN (polyacrylonitrile) and pitch (petroleum oil and coal)
Mechanical strength, modulus of elasticity, heat resistance, and chemical resistance are all advantages of the fibers. They have similar electric resistance and heat conductivity to metals. As a result of their low thermal expansion coefficient, they are used to make electromagnetic shields, electrodes, and heat-resistant structures.
Alumina Fiber
These are ceramics made by spinning a slurry of alumina particles and additives into a yarn and then heating it under regulated conditions. At high temperatures, fibers maintain their strength. At high temperatures, it also has good electrical insulation. It has a high hardness and wear resistance.
• Silica fibers are sodium silicate fibers (water glass). They're employed in heat protection (asbestos replacement) as well as packings and compensators. They can be manufactured to be almost completely free of non-alkali metal complexes.
After that, sodium silicate fibers can be used to make silica fibers, which is preferable than making them from a melt containing SiO2 or by acid-leaching glass fibers. Wet webs, filter linings, and material reinforcement can all benefit from silica fibers.
They can also be utilized to make silicic acid fibers using a dry spinning process. These fibers possess qualities that make them suitable for use in friction-reducing materials. 1st
Boron Fibers
PMCs and, to a lesser extent, MMCs are reinforced with boron fibers. Boron fibers are made as monofilaments (single filaments) by CVDing boron onto a tungsten wire or a carbon filament, with the latter being the most common. In comparison to most other reinforcements, they have rather enormous diameters (100–140 m). Table 3 shows characteristic parameters of tungsten-cored boron fibers with a diameter of 140 m. Effective fiber qualities are influenced by the ratio of overall fiber diameter to tungsten core diameter. Fiber specific gravity, for example, is 2.57 for 100 m fibers and 2.49 for 140 m fibers. Because boron fibers are more expensive than many forms of carbon fibers, they are used in far fewer applications.
1.10.1 Whisker reinforcements
The table below shows the bulk and whisker properties of several materials. The table shows that the gap in bulk and whisker strengths is quite large.
Table 2.2: Properties of Some Common Engineering Materials in Bulk and Whisker Forms
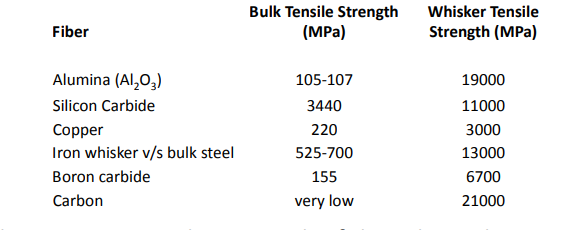
Fibers and whiskers are one of the constituent components in modern composites, and they provide many of the required physical features.
1.10.2 Particulate reinforcements
Because of their low cost, particle reinforcements have been frequently utilized to improve the characteristics of polymeric matrixes. Particles can be easily blended with polymers in powder form for the SLS process, liquid form for the SLA process, and even extruded into printable filaments for the FDM process (Wang et al., 2017). The development of PMCs addresses and resolves commercialization and real-world uses of polymers. Hwang et al. (2015) recently investigated the thermo-mechanical characteristics of metal reinforced PMCs using the FDM technique. Copper and iron particles with average sizes of less than 24 and 43 m, respectively, were used. The effects on the characteristics of the 3D printed composite were studied using metallic particle reinforcements in various concentrations ranging from 10% to 80%. The viscosity was lowered while the tensile strength was raised by raising the temperature during extrusion from 190°C to 220°C. Furthermore, increasing the reinforcement content boosted ductility, but when the filler level above a certain threshold, ductility began to drop (Chung, 2001).
The physical, chemical, and mechanical properties of the polymeric matrix can all be improved by the presence of particle reinforcements. Furthermore, the inclusion of reinforcement particles can help to resolve several issues that arise during the printing process. Because monolithic polymers have a significant thermal expansion, poor bonding between layers is a major challenge in the printing process. Metallic particles can aid to reduce the thermal expansion of the final composite and improve the bonding between the layers (Chung, 2001).
The use of various hydrogel compositions (e.g., alginate, chitosan, and gelatin) in 3D printing of cell-laden scaffolds reinforced with HAp particles has been studied (Kesti et al., 2015; Wenz et al., 2017). The results reveal that adding HAp particles to hydrogels significantly improves their mechanical properties and promotes osteogenic differentiation in vivo, making them suitable for mending bone tissue defects (Wenz et al., 2017; Bendtsen et al., 2017).
Nikzad et al. (2011) investigated the impact of copper and iron particles reinforcing the ABS matrix. The results revealed that the presence of iron particles reduced ABS tensile strength, resulting in a composite that was tougher and more brittle than the matrix. Masood and Song et al. Created a metallic powder reinforce PMC by inserting iron particles in the P301 nylon matrix with diameters ranging from less than 30 to 80 m. (Masood and Song, 2004). The composite that emerged was found to be suitable for direct quick tooling applications. It was also discovered that the mechanical qualities of the 3D printed product can be harmed by reinforcements with greater particle sizes. In other words, it was discovered that particulate reinforcements with lower particle sizes could improve the polymeric matrix's characteristics. The use of nanoparticles to improve PMC characteristics will be described in the next subsections.
1.11.1 Reinforcement – matrix interface
The matrix is a completely continuous monolithic material into which the reinforcement is embedded. Unlike two materials sandwiched together, there is a channel through the matrix to any location in the material. The matrix is commonly a lighter metal, such as aluminium, magnesium, or titanium, in structural applications, and offers a compliant support for the reinforcement. Cobalt and cobalt-nickel alloy matrices are used in high-temperature applications.
The matrix constituent is a typical way to classify composite materials. Organic Matrix Composites (OMCs), Metal Matrix Composites (MMCs), and Ceramic Matrix Composites are the three basic composite classifications (CMCs). The term "organic matrix composite" is widely used to refer to two types of composites: polymer matrix composites (PMCs) and carbon matrix composites, often known as carbon-carbon composites.
Three common forms of composites result from these three sorts of matrices.
1. Ceramic fibers are used in a plastic matrix in polymer matrix composites (PMCs), of which GRP is the most well-known example.
2. Silicon carbide fibers are commonly inserted in an alloy of aluminium and magnesium in metal-matrix composites (MMCs), but other matrix materials such as titanium, copper, and iron are increasingly being employed. Bicycles, golf clubs, and missile guidance systems are all examples of MMC applications; an MMC constructed of silicon carbide fibers in a titanium matrix is now being researched for use as the skin (fuselage material) of the US National Aerospace Plane.
3. Ceramic-matrix composites (CMCs) are the third major category, with silicon carbide fibers set in a borosilicate glass matrix as an example. They are particularly well suited for usage in lightweight, high-temperature components, such as parts for aeroplane jet engines, due to their ceramic matrix.
Several factors influence the selection of a matrix alloy for an MMC. It's crucial to know if the composite will be reinforced constantly or intermittently. When continuous fibers are used as reinforcements, the majority of the load is transferred to the reinforcing filaments, and composite strength is mostly determined by fiber strength. The matrix alloy's principal functions are to transport load efficiently to the fibers and to soften cracks in the case of fiber failure, hence the matrix alloy for continuously reinforced composites may be chosen for toughness rather than strength.
In continuously reinforced composites, lower strength, more ductile, and harder matrix alloys may be used. The matrix may control composite strength in discontinuously reinforced composites. The choice of matrix will then be determined by the necessary composite strength, which may necessitate the use of higher strength matrix alloys. Potential reinforcement/matrix reactions, either during processing or in service, which could result in degraded composite performance; thermal stresses due to thermal expansion mismatch between the reinforcements and the matrix; and the influence of matrix fatigue behaviour on the cyclic response of the composite are all factors to consider when choosing the matrix. Indeed, the behaviour of composites under cyclic loading situations is something that needs to be taken into account. The difference in melting temperatures between the matrix and the reinforcements is an additional issue in composites intended for use at high temperatures. Even at temperatures near the matrix melting point, a substantial melting temperature differential can cause matrix creep while the reinforcements remain elastic. When there is a tiny melting point difference in the composite, however, creep in both the matrix and reinforcement must be considered.
1.11.2 Wettability
WETTABILITY IS THE preference of a liquid to be in contact with a solid surrounded by another fluid (liquid or gas). Depending on the application, wettability can be wanted or not. Take for example a newly waxed car. The purpose of adding the wax is to prevent the water from spreading and to prevent corrosion of the car. In this case, the aim is clearly to reduce the wettability. Then, on the other hand, good wettability is needed when for example coating is applied on the surface (wax on top of the car).
Key takeaways:
- The matrix binds the fiber reinforcement, transfers loads between fibers, gives the composite component its net shape and determines its surface quality. A composite matrix may be a polymer, ceramic, metal or carbon
- Following are the functions of the reinforcement in a composite: It increases the mechanical properties of the composite. It provides strength and stiffness to the composite in one direction as reinforcement carries the load along the length of the fiber.
- The matrix is basically a homogeneous and monolithic material in which a fiber system of a composite is embedded. It is completely continuous. The matrix provides a medium for binding and holding reinforcements together into a solid.
In a wide range of industries, such as construction, automotive, and aerospace, the adhesion and corrosion resistance of polymer/oxide/metal interphases in functional composite materials is an enormously significant component of engineering. To protect the functional qualities of the composite, interphases in polymer/metal junctions must survive strong mechanical pressures and corrosive attack over long periods of time. Factors such as alloying element enrichment and the presence of intermetallic precipitates at the metal-polymer interface are well known to have a significant impact on the corrosive de-adhesion of organic layers. Despite decades of research, the adhesion between metals and polymers (organic coatings and adhesives) as well as the loss of adhesion in the presence of an aqueous environment are still under investigation.
The complex interaction of: - Molecular forces at the interface (chemical bonding, van der Waals bonding, hydrogen bonding, acid-base interactions, etc.) determines adhesion and de-adhesion processes.
- Within the interphase, the polymeric chains are arranged supramolecularly.
- The metal substrate's surface chemistry
- The metal oxide layer's, interphases, and bulk polymer phase's mechanical properties
- Within the interphase, the defect density
- The metal oxide's and surrounding polymer's electrical characteristics
- The interphase's hydrolytic and oxidative resistance
- The interphase's barrier qualities
In both dry and humid settings, molecular forces at polymer/oxide/metal interfaces control the adhesion inside the contact. The molecular adhesion forces, which act only over a distance of 2-3 nanometers, are influenced by disperse and polar forces, hydrogen bonding, acid-base interactions, and covalent bonding. The chemistry of the oxide, as well as the chemistry and supramolecular ordering of the adsorbed polymer, influence the forces that arise. If the polymer/metal junction is exposed to moisture and/or ion-containing electrolytes under ambient circumstances, the mobility of water, hydrated ions, and electrons affect the kinetics of adhesion loss and corrosive de-adhesion. The intense adsorption of water molecules on oxides and the resulting induced replacement of adsorbed polymeric chains causes a decrease in adhesion force in the presence of moisture. The inclusion of hydrated ions causes a significant potential drop at the buried interface, which increases electrochemical reactions due to their mobility along the interface between the oxide covered metal and the polymer. The kinetics of oxygen reduction at the interface are determined by electron transport from the metal through the oxide to adsorbed oxygen molecules. It is critical to conduct both a fundamental analysis at the molecular level using model molecules that simulate the functional groups of organic coatings and a macroscopic examination of adhesion and corrosive de-adhesion capabilities on a variety of customized base metal/metal oxide surfaces. The nature and kind of base metallic substrate depends on the application areas; for example, special aluminium alloys and galvanized steels are of special relevance in the aerospace and automobile industries, respectively. The examination of the physico-chemical properties of complex polymer interfaces, the application of advanced spectroscopic methods, and the theoretical computation of transport phenomena in thin films can all be used to evaluate transport phenomena.
You're looking for a new glue and notice that the most recent alternative on the market claims to have unrivalled binding strength. You're definitely interested because adhesive strength and reliability can make or break the success of a restoration. But what does it mean when the firm claims the product has the strongest bond strength, and how did they assess it?
While the figures are appealing, it's critical to understand where they come from. There are a variety of bond-strength testing methods, each of which operates differently and yields different findings.
Dr. John Flucke, DPR's technology editor who practices in Lee's Summit, Mo., adds, "Bond strength is a phrase and process that may be quantified in many different ways." “It's critical to understand how bond strength estimates from a manufacturer were calculated if you're going to depend on them.”
To measure bond strength, there are two types of testing (static and dynamic), although within these categories, there are multiple approaches, each with its own set of strengths and weaknesses. It's helpful to know which approach was used when evaluating adhesive bond strength so you can compare goods from different manufacturers on the same scale.
Static tests
The more common of the two methods of testing is static testing, which involves applying a load to a test specimen while it is stationary. There are two types of static tests: macro tests (when the bond area is greater than 3 mm2) and micro tests (where the bond area is less than 3 mm2) (where the bond area is less than 3 mm2, and is usually 1 mm2 or less). 1
Macro testing
Macro testing is divided into three main methods: shear, tensile, and push-out. It is known for its simplicity.
1.13.1 Macro-shear bond strength (SBS) testing
The most frequent method for evaluating the bond strength of new adhesives is macro-shear bond strength testing. In shear bond tests, two materials are bonded using an adhesive and loaded in shear until it fractures.
SBS testing is the simplest and fastest type of testing since it requires no further specimen processing or preparation once the materials are bonded. However, there are issues with measuring SBS since it places a lot of stress on the bonded elements (such as the tooth substrate), which can break before the glue. As a result, it can cast doubt on the outcomes (did the adhesive fail, or was it the tooth?).
1.13.2 Macro-tensile bond strength (TBS) test
The TBS test is less popular than the SBS test, but it is useful for determining the bond strength of cements to hard materials such metal alloys and ceramics. A specimen is put into a mechanical testing machine and clamped into place for TBS tests. The specimen is then loaded perpendicularly on both sides to prevent it from bending (if the specimen is placed horizontally, bending stress will quickly develop).
TBS testing has the advantage of having a more uniform distribution of stress than shear tests. As a result, the test can provide a more precise estimate of the stress level that causes bonds to rupture.
1.13.3 Push-out (PO) test
Push-out tests are used to determine the fatigue resistance of adhesive-dentin bonds, especially in the case of posts luted in root canals and root-canal sealers. Researchers use this technology to punch a tapered cylindrical hole in a 1-2mm thick dentin slice. After applying adhesive to the inside of the hole, it is filled with composite material. “The composite cylinder is then forced through the dentin from the smaller diameter side, and the bond strength is measured by dividing the extrusion force by the lateral area of the tapered cylinder,” El Mourad noted in a 2018 study.1
Because the composite is extracted parallel to the cement and dentin interface, these tests provide more accurate information than shear testing because failure occurs parallel to the cement and dentin interface. PO tests, on the other hand, aren't very popular because specimen preparation and technique take a long time, and the results are difficult to duplicate.
Micro testing
Although many tests are identical in execution to macro tests, micro testing enables for examination of small dental regions. Micro testing is separated into three procedures, similar to macro testing: micro shear, micro tensile, and micro push-out.
1.13.4 Micro-Shear Bond Strength (µSBS) Test
SBS testing, which was first launched in 2002, uses tiny samples to allow for the fabrication of several specimens from the same tooth. This ensures a more consistent approach to testing and eliminates variables that could influence the conclusion. It is commonly used to test glass ionomers, enamel, and other qualities that are too sensitive for micro-tensile bond testing stresses. 1
While SBS testing is identical to regular SBS testing, it has been proven to be less effective at measuring bond strength.
1.13.5 Micro-tensile bond strength (µTBS) test
µTBS Testing is quite effective and provides many benefits that shear tests do not, but it comes at a price: It's highly technique-sensitive and labor-intensive because it necessitates substantial specimen preparation.
Adhesive resins are attached to the entire flat occlusal surface of a tooth in TBS testing, and subsequently the tooth is covered in resin composite. After curing and storing the sample in water, it is vertically sectioned with a slow-speed diamond saw. This shows the composite, glue, and dentin in cross section. Because this methodology distributes stress more evenly at the contact, failure occurs only at the adhesive connection, rather than on other surfaces as in shear tests. While it generally produces reliable findings, it is not without flaws.
µTBS testing is not reliable when measuring bond strengths less than 5 MPa.
1.13.6 Micro push-out (µPO) test
This modification of the traditional PO test has not been investigated as fully as other approaches. Typically used to measure the bond strength of luted fiber posts, it uses dentin disks that are 1 mm or less in thickness. µPO tests usually result in higher values than normal push-out methods, so its accuracy is still undetermined.
Dynamic tests
Because a bond is rarely subjected to stationary loading, dynamic testing more properly mimics stress that may occur therapeutically. As a result, fatigue tests are thought to be a better predictor of adhesives' long-term performance. They're mostly used in dentin bonding because enamel bindings are significantly more difficult to evaluate.
Dynamic testing is uncommon, despite the fact that they are more time consuming and labor-intensive than static-bond strength testing. There is currently no standard for adhesive fatigue testing, making it difficult to quantify and replicate. 3 Numerous forms of fatigue testing have been reported because to the lack of a standard, including macro- and micro-shear, macro push-out, micro-rotary, micro-tensile, and micro-3- and 4-point bend procedures, with 3- and 4-point bending tests being the most popular.
1.13.7 Factors that can affect testing
Other factors, in addition to the procedures for determining bond strength, can influence the findings of bond-strength testing.
The makeup of the teeth is important. Cow teeth are frequently utilized in place of human teeth in tests because they are easier to obtain. However, biological changes in bovine dentition, such as larger dentinal tubules and dentin closer to the pulp, can influence the test's accuracy in human teeth. However, even within human teeth, the type of tooth analyzed can have an impact on test results. Using third molars (which are more permeable than erupted teeth) or teeth with pre-existing carious lesions, for example, can alter the bond-strength testing results. Bond strength diminishes with increased surface area, hence the size of the specimen can affect the results.
Another consideration is dentin depth, as adhesives have higher bond strengths in the surface dentin and decreasing bond strengths as the dentin gets deeper. Additionally, factors such as pulpal pressure, composite characteristics, mechanical testing equipment and design, and operator error can all affect test results.
Even if these variables could be avoided and a consistent testing process implemented, lab testing has limitations in terms of what it can reveal. While determining immediate bond strength (and potential reducing effectiveness) is relatively simple, evaluating long-term bond strength (and potential reducing efficacy) is more difficult. Although ageing factors (such as water storage, mechanical loading, enzyme breakdown, or thermocycling) are used in lab testing, they cannot reproduce the real-world pressures that bonding agents face. As a result, laboratory findings may not necessarily correspond to clinical behaviour. 1
According to Sridhar Janyavula, BDS, MS, a clinical research dentist at DENTSPLY Caulk, “most of the new generation of bonding agents (such as universal adhesives) perform satisfactorily on the bench tests.” “The most important issue doctors should ask is what evidence is available to assess a material's long-term effectiveness beyond the 24-hour lab testing that most manufacturers provide. With the scant data available, it's possible that the 'too good to be true' adhesives that claim to bond to anything on the planet have shown decreased binding strengths over time in ageing experiments.”
1.13.8 Evaluation of testing
While bond strength is an important aspect in adhesive performance, it isn't the sole characteristic to consider when evaluating the efficacy of bonding agents. Bond-strength analyses should be integrated with results from microleakage testing, bond durability testing, and gap evolution studies, according to the researchers.
If you choose an adhesive solely based on bond strength, keep in mind that not all bonding tests are created equal, and there is no standardized testing technique for measuring standard binding strength. Finally, testing procedures must be standardized and standard in order for bond-strength tests to be valid and provide concrete information to practitioners.
References:
1. Composite Materials: Engineering and Science, by Matthews and Rawlings, CRC Press.
2. An Introduction to composite material, by D.Hull and T.W. Clyne, Cambridge University press.
3. Metal Matrix Composites, Thermomechanical Behaviour by M.Taya, and R.J.Arsenault, Pergamon Press, Oxford.
4. Fundamentals of Metal Matrix Composites by S.Suresh, A.Martensen, and A.Needleman, Butterworth, Heinemann
5. Mechanics of composite materials, R. M. Jones, Mc Graw Hill Book Co.