Unit – 2
Metal matrix composites
2.1.1 Metal Matrix Composites (Introduction)
• A Metal Matrix Composite (MMC) is a material made up of a metallic matrix and a ceramic (oxides, carbides) or metallic (lead, tungsten, molybdenum) distributed phase, according to the categorization of composite materials.
• Composites using Aluminum Matrix (AMC)
• Composite Magnesium Matrix
• Composite Titanium Matrix
• Composites with a Copper Matrix
• Some Metal Matrix Composites' Properties
2.1.2 Aluminum Matrix Composites (AMC)
• This category contains the most Metal Matrix Composites. Aluminum Matrix Composites' matrices are often made of aluminum-silicon (Al-Si) alloys, particularly those in the 2xxx and 6xxx series. • Alumina (Al2O3) or silicon carbide (SiC) particles (particulate Composites) in amounts of 15-70 vol percent; • Continuous fibres of alumina, silicon carbide, or graphite in amounts of 15-70 vol percent; (long-fiber reinforced composites)
• Alumina fibres that are not continuous (short-fiber reinforced composites); The following fabrication procedures are used to make Aluminum Matrix Composites:
• Sintering (powder metallurgy);
• Casting with a stir;
• Surveillance. Aluminum Matrix Composites have the following characteristics:
• High tensile strength, even at high temperatures;
• High stiffness (elasticity modulus);
• The density is low;
• Excellent thermal conductivity;
• Excellent resistance against abrasion.
Automotive parts (pistons, pushrods, brake components), brake rotors for high-speed trains, bicycles, golf clubs, electronic substrates, and cors for high-voltage electrical cables are all made with Aluminum Matrix Composites (AMC).
2.1.3 Magnesium Matrix Composite
• Silicon carbide (SiC) particles are used to strengthen magnesium matrix composites (particulate composites) Magnesium Matrix Composites have the following characteristics:
• Low density; • High stiffness (elasticity modulus);
• Excellent wear resistance;
• Excellent tensile strength, even at high temperatures;
• Excellent creep resistance.
• Magnesium Matrix Composites are utilised in the production of racing vehicle components, lightweight automotive brake systems, and aircraft equipment such as gearboxes, transmissions, compressors, and engines.
2.1.4 Titanium Matrix Composite
• Continuous monofilament silicon carbide fibre (long-fiber reinforced composites); • Titanium boride (TiB2) and titanium carbide (TiC) particles are used to reinforce titanium matrix composites (particulate composites). Titanium Matrix Composites are made via powder metallurgy (sintering). Titanium Matrix Composites have the following characteristics:
• Extremely durable;
• High stiffness (elasticity modulus);
• Excellent creep resistance;
• Excellent thermal stability;
• Excellent wear resistance.
Titanium Matrix Composites are used to make structural elements for the F-16 jet's landing gear, turbine engine components (fan blades, actuator pistons, synchronisation rings, connecting links, shafts, and discs), automobile engine components, drive train elements, and general machine components.
2.1.5 Copper Matrix Composites
• Continuous carbon (**C**), silicon carbon (SiC), tungsten (W), and stainless steel 304 (long-fiber reinforced composites) fibres are used to strengthen Copper Matrix Composites (particulate composites). Copper Matrix Composites are made using powder metallurgy (sintering) and an infiltration process. Copper Matrix Composites have the following characteristics:
• Low thermal expansion coefficient;
• High stiffness (elasticity modulus);
• Electrical conductivity is good
• Excellent thermal conductivity;
• Excellent wear resistance.
Hybride modules, electronic relays, electrically conducting springs, and other electrical and electronic components are made with Copper Matrix Composites.
2.1.6 Properties of some Metal Matrix Composites
- Metal Matrix Composite MC-21
- Metal Matrix Composite Duralcan F3S.20S
- Metal Matrix Composite 2124-25%SiC
- Metal Matrix Composite Al-60% Al2O3 fiber
- Metal Matrix Composite Al-2%Cu-60% Al2O3 fiber
Key takeaways:
• Composition is important. A reinforcing substance is dispersed into a metal matrix to create MMCs. To avoid a chemical reaction with the matrix, the reinforcing surface can be coated. Carbon fibres, for example, are frequently utilised in an aluminium matrix to create composites with low density and great strength.
• MMCs, particularly aluminium alloys with reinforced particles, are widely employed as structural materials in high-tech fields such as aerospace, defence, automotive, and civil engineering.
• Aluminum MMCs are made from aluminium that has been reinforced with ultra-fine ceramic particles to improve strength and wear resistance.
2.2.1 Metal-Matrix Composites
For the Space Shuttle, commercial airliners, electronic substrates, bicycles, automobiles, golf clubs, and a number of other uses, metal-matrix composites are either in use or in prototyping.
For the Space Shuttle, commercial airliners, electronic substrates, bicycles, automobiles, golf clubs, and a number of other uses, metal-matrix composites are either in use or in prototyping. While aluminium matrix composites account for the great majority of applications, a rising number of them require the matrix qualities of superalloys, titanium, copper, magnesium, or iron.
Aluminum-matrix composites, like other composites, are a group of materials that can be adjusted in terms of stiffness, strength, density, and thermal and electrical properties. To obtain the desired qualities, the matrix alloy, reinforcement material, reinforcement volume and form, reinforcement site, and fabrication method can all be changed. Regardless of the differences, aluminium composites have the advantage of being less expensive than most other MMCs. They also have good thermal conductivity, high shear strength, good abrasion resistance, high-temperature operation, nonflammability, low fuel and solvent attack, and the ability to be formed and processed on conventional equipment.
Casting, powder metallurgy, in situ reinforcement development, and foil-and-fiber pressing processes are used to make aluminium MMCs. With major producers ramping up production and lowering prices, consistently high-quality items are now available in big quantities. They're used in golf clubs, bicycles, machinery components, electronic substrates, extruded angles and channels, and a range of other structural and electronic applications, in addition to brake rotors, pistons, and other automotive components.
For components in jet turbine engines that run at temperatures above 1,830 degrees Fahrenheit, superalloy composites reinforced with tungsten alloy fibres are being developed.
Graphite/copper composites have tailorable features, are helpful in high-temperature environments, and have outstanding mechanical, electrical, and thermal conductivity. When compared to titanium, they are easier to work with and have a lower density than steel. Ductile superconductors were created using a copper matrix and niobium-titanium superconducting filaments. Heat sinks and electronic packaging are made of copper enhanced with tungsten or aluminium oxide particles.
The skin material for the National Aerospace Plane is titanium reinforced with silicon carbide fibres. The matrix materials reinforced with titanium carbide particles and fabricated into draw-rings and other high-temperature, corrosion-resistant components include stainless steels, tool steels, and Inconel.
2.2.2 Compared to monolithic metals, MMCs have:
• Higher density-to-strength ratios
• Stiffness-to-density ratios that are higher • Fatigue resistance that is better
• Better characteristics at high temperatures
o — Increased power
o — Reduced creep rate
• Lower thermal expansion coefficients
• Improved wear resistance
MMCs have the following advantages over polymer matrix composites:
• Capability to withstand higher temperatures
• Resistance to fire
• Stiffness and strength in the transverse direction are improved
• There is no moisture absorption.
• Electrical and thermal conductivities that are higher
• Increased resilience to radiation
• There is no outgassing
• The ability to fabricate whisker and particulate-reinforced MMCs using standard metalworking equipment.
MMCs have a number of drawbacks when compared to monolithic metals and polymer matrix composites, including:
• Some material systems have a higher cost.
• Technology that is still in its infancy
• Fiber-reinforced systems require complex fabrication procedures (except for casting)
• No prior service experience
Since the late 1950s, when work on MMC began, a variety of matrices and reinforcements have been attempted. However, MMC technology is still in its infancy, and more significant systems will probably arise.
2.3.1 Processing of Metal Matrix Composites
Processing of metal matrix composites (MMC) can be classified into three main categories: -
1) Solid State Processing
2) Liquid State Processing
3) In-Situ Processing
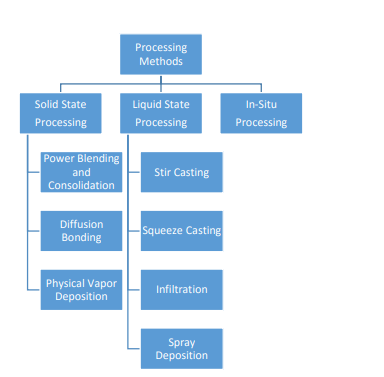
1) Solid State Processing
The main fabrication methods for solid state processing of metal matrix composites are powder blending and consolidation, and physical vapor deposition.
a) Powder Blending and Consolidation:
Metal alloy powder is blended with ceramic whisker/short fiber/particles in dry condition or in liquid suspension. After blending, the mixture is further processed by cold compaction, canning, degassing, and high temperature consolidation. There are some oxide particles in volume fraction of 0.05-0.5 depending on the powder and processing conditions that help Processing Methods Solid State Processing Power Blending and Consolidation Diffusion Bonding Physical Vapor Deposition Liquid State Processing Stir Casting Squeeze Casting Infiltration Spray Deposition In-Situ Processing dispersion-strengthening of the metal matrix composites . This method is usually used for the processing of aluminum and magnesium metal matrix composites.
b) Diffusion Bonding:
The inter diffusion atoms at the metallic surfaces under pressure creates bonding between the metal matrix and fibers [14]. This fabrication method is widely used for aluminum or magnesium MMCs reinforced with continuous/discontinuous fibers.
c) Physical Vapor Deposition:
Fibers are carried through an area with a high partial pressure of the metal to be deposited on a continuous basis. The vapour is created and introduced into the process, and then condensation occurs in this area, resulting in a fibre coating. Deposition occurs at a rate of 5-10 micrometres per minute. Hot pressing or hot isostatic pressing is used to consolidate the coated fibres.
2. Liquid State Processing
a) Stir Casting: Particulate reinforcements are combined with liquid metal melt, which solidifies the combination. A revolving impeller creates a vortex of molten alloy, and the pre-treated particles are injected into that vortex. The reinforcements are not consistently dispersed throughout the stir casting process, resulting in sediments in the molten alloy. In general, metal alloys can contain up to 30% particles with a diameter of 5 to 100 micrometres. Al-(10-15%) B4C MMCs are an example of this approach. Particles are inserted into the metal alloy in a semi-solid condition in another variation of the stir casting procedure.
b) Squeeze Casting
An open die is filled with molten metal. The dies are then closed, allowing the molten metal to solidify within the dies under pressure. Under high pressure and through contact between the metal and the die surface, heat is rapidly transmitted from the molten metal to the dies. As a result, this process produces a fine-grain casting with little or no porosity.
c) Process of Infiltration:
The porous shapes of fibers/whiskers reinforcements are infiltrated with liquid metal alloy. Depending on the level of porosity, the volume proportion of the reinforcements normally ranges from 10 to 70%. Binders such as silica and metal-based combinations are frequently used to maintain the integrity and shape of porous structures
d) Deposition of Spray
Injecting particle/whisker/short fibre reinforcements into the spray results in a porosity deposition layer of 5-10% on the metal surface. The depositions are then further processed to achieve full density. Matrix metals are sprayed onto the fibres in continuous (long) fibre reinforced metal matrix composites. In this processing procedure, the fibre spacing and fibre layer have an impact on the fibre volume fraction and distribution.
3. In-Situ Processing
In-situ processing involves chemical reactions that result in the creation of reinforcing phase within a metal matrix. The reinforcements can be formed from the precipitation in liquid or solid. This method provides thermodynamic compatibility at the matrixreinforcement interface. The reinforcement surfaces are also likely to be free of contamination and, therefore, a stronger matrix-dispersion bond can be achieved.
2.4.1 Diffusion Bonding
Diffusion bonding is one of the various vacuum processes to be aware of today (aka diffusion welding or thermo-compression bonding). Despite its specialist nature, this technology continues to gain appeal among design engineers and is finding increasing uses in manufacturing. Let's find out more.
A solid-state bond is a way of joining two or more mating pieces together that does not require the use of an interface layer (i.e., without the application of a material between the parts applied by plating, sputtering, ion implanting or brazing or in the form of a foil). The resulting interface must be bonded at or below the melting point of either the parent material or any formed eutectic (i.e., Tbond Tmelting point).
A 50 percent or more increase in grain size is typical. To produce a high-quality hermetic seal, the total part strain must be in the range of 2-4 percent. Because of the strain requirement, a significant load (i.e., unit-normal force) must be supplied to the bonding surfaces to produce adequate results.
2.5.1 Introduction to Powder Metallurgy
What is powder metallurgy, and how does it work? Powder metallurgy is a metal-forming technique in which compacted metal powders are heated to just below their melting temperatures. Despite the fact that the process has been around for more than a century, it has only recently gained widespread acceptance as a superior method of making high-quality parts for a range of critical applications. This success can be attributed to the technique' benefits over other metal forming methods like forging and metal casting, such as material utilisation, shape complexity, and near-net-shape dimensional control, among others. Powder metallurgy is a renowned green technology because of these factors, which contribute to sustainability.
Figure 1 shows a complicated planetary carrier for a four-wheel drive torque transfer system, a helical gear and stainless steel blades used in laparoscopic surgical scissors, an offshore oil platform manifold weighing over 6.5 tonnes, and a steel connecting rod used in V-8 engines. Powder metallurgy was used to create all of these parts.
2.5.2 Liquid state fabrication of Metal Matrix Composites
It entails incorporating a dispersed phase into a molten matrix metal and then solidifying it.
Good interfacial bonding (wetting) between the dispersed phase and the liquid matrix is required to achieve high mechanical characteristics in the composite.
Coating the dispersed phase particles can help to improve wetting (fibers). Coating decreases interfacial energy while also preventing chemical interactions between the dispersed phase and matrix.
2.5.3 Metal stirring
Metal stirring in a coreless induction furnace had been a mystery until Inductotherm's research in the mid-1970s revealed the science behind the stirring forces. Until that moment, it was assumed that inductive stirring in an induction furnace was proportional to the furnace's meniscus height.
Meniscus height is proportional to kilowatts and inversely proportional to frequency squared. However, it became clear during actual melting operations that this simplified methodology did not provide an appropriate measure of stirring. Even though the meniscus heights were the same, furnaces holding the same amount of the same metal but running at different frequencies did not stir the same.
Key takeaways:
• Powder metallurgy is the process of combining fine powdered materials, compacting them into a desired shape or form, and then heating the compressed material in a controlled atmosphere to bond it (sintering).
• Part-to-part consistency for enhanced product quality, shape and material flexibility, application versatility, and cost effectiveness are all advantages of the powder metallurgy process over competing metalworking methods.
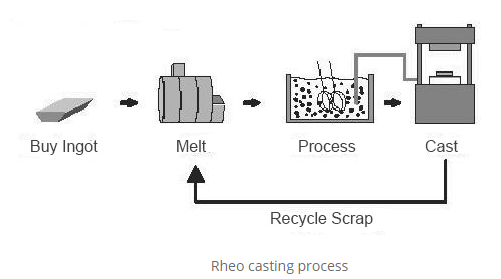
2.6.1 The Rheo Casting Process
Within the context of foundry technology, defects and anomalies are a daily challenge. The issue of quality becomes more critical as demand for castings with highly specialised applications grows.
The Rheo Casting process uses a semi-solid slurry, with the amount of advantages being directly proportional to the fraction solid at the moment of casting. A reduction in shrinkage and a large reduction in latent heat are possible benefits.
Defects, abnormalities, and faults in the final product are an unavoidable reality in the context of foundry technology. Because of the demand for better casting performance in order to create a high number of components with essential applications, this cohabitation between process and quality difficulties is becoming increasingly difficult.
During solidification, voids or cavities are created within a casting because to volume contraction, inefficient feeding systems, and/or gas (most commonly hydrogen) formation. Independent of the stress circumstances, interdendritic shrinkage pores, inclusions, and secondary dendrite arm spacing are favoured crack starting locations. These variables have a direct impact on the alloy's mechanical properties, resulting in lower strength and ductility, irregular fracture growth, and, in severe cases, material failure.
SSM-processing is a new manufacturing method for aerospace, military, and, in particular, automotive components. Suspension parts, engine brackets, and fuel rails for the automobile sector are made in Europe, whereas mechanical parts for snowmobiles and mountain bikes are made in the United States. With an emphasis on magnesium alloys, Asia has focused more on the production of electronic components such as electrical housing components and notebook chassis.
Rheocasting entails making an SSM slurry straight from the liquid alloy, then shaping it using a method like High Pressure Die Casting (HPDC). The alloy is cooled to a semi-solid condition and then put into a die without an intermediary solidification phase in "Rheo" procedures, resulting in a semi-solid slurry with non-dendritic solid particles from a fully liquid regular alloy. It is then cooled to the necessary fraction solid before being cast into a component. Component shaping directly from SSM slurries is intrinsically appealing because to features like overall production efficiency and energy management.
The ability to cast metal at a wide range of fraction solids is a key advantage of rheocasting. The majority of the process benefits of nondendritic, semi-solid alloys are proportional to the amount of solid present at the time of casting. Reduced shrinkage, less latent heat, and the degree of viscosity are all dependent on the percentage of solid in the alloy being increased.
Semi-solid casting, on the other hand, begins to vary from traditional die casting techniques as the solid proportion grows. Because of the much higher viscosity of the alloy, a more powerful shot end on the die cast machine is necessary for the larger percent solid material. In order to fit the wider hole in the cold chamber, the piston's stroke is normally longer. As a result, while high percentage solid casting reduces casting cycle time, it also necessitates more costly adjustments to the die casting process to accommodate the more viscous material.
The schematic overview of the rheo casting method and microstructure of rheocast A356 alloy is shown in Figures 1 and 2.

The molten metal is put into the pre-heated die's bottom half. The upper part closes the die and adds pressure during the solidification process when the metal begins to harden. The amount of pressure utilised is substantially less than that required in forging, allowing for the production of intricate pieces. This procedure can be used to create holes and recesses using coring. Porosity is low, and mechanical characteristics are better.
This process can be used to make ferrous and non-ferrous materials.
Squeeze casting is a technique for shaping metal into shapes by squeezing two dies together. Unlike most casting procedures, which utilise two dies that are squeezed together before the metal is added, squeeze casting uses two dies that are pressed together after the metal has been introduced. The upper die is only removed after the liquid metal has cooled. The metal will normally come out stronger, with a nicer grain and less metallic shrinkage, if this process is used. Magnesium, aluminium, and their alloys are often utilised, although many other metals can be utilised as well.
In most casting procedures, two dies are used, but squeeze casting uses the dies in a unique method. Normally, the two casts are joined and liquid metal is poured into the casing. A pool of liquid metal is deposited in the bottom die, and a top die comes in and squeezes the metal into a shape using a squeeze cast. Because pressure is exerted via the upper die, this is not strictly casting, but rather a hybrid process that incorporates forging.
2.7.1 Squeeze casting process
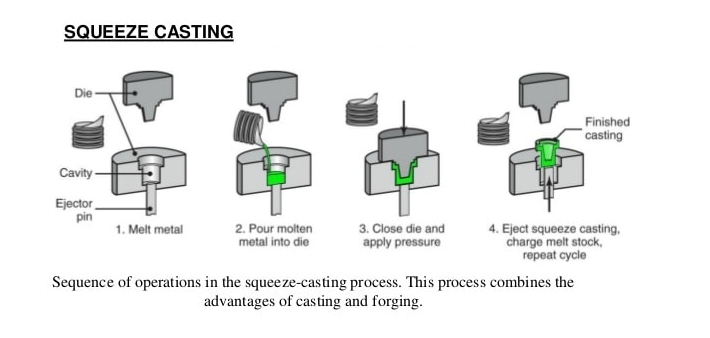
2.7.2 Squeeze casting process
Only liquid metal can be used in this application. While materials such as plastic can melt at high temperatures, this technique will not be suitable to cast plastic. After the upper die is set, workers wait until the metal is completely cool. Once cool, the upper die will be released and the required shape will have been cast into the now-solid metal.
Non ferrous alloys like aluminum, magnesium, and copper alloy components are readily manufactured using this process . The squeeze casting process, combining the advantages of the casting and forging processes, has been widely used to produce quality castings . Because of the high pressure applied during solidification, porosities caused by both gas and shrinkage can be prevented or eliminated . The cooling rate of the casting can be increased by applying high pressure during solidification, since that contact between the casting and the die is improved by pressurization, which results in the foundation of fine-grained structures
Key takeaways:
- Squeeze casting, also known as liquid forging, is a hybrid metal forming method that combines permanent mould casting and die forging in a single step by pouring a specified amount of molten metal alloy into a preheated and greased die and then forging and solidifying it under pressure.
- Composition of the casting alloy, applied pressure level, die preheating temperature, pouring temperature, die coat material (lubricant), melt superheat, duration of pressure application, punch temperature, and delay time to achieve maximum pressure have all been identified as variables that affect squeeze casting.
Gas Pressure Infiltration is a forced infiltration method of Metal Matrix Composites liquid phase manufacturing that uses a pressurised gas to impart pressure to molten metal and compel it to enter into a prepared dispersed phase.
2.8.1 Gas Pressure Infiltration
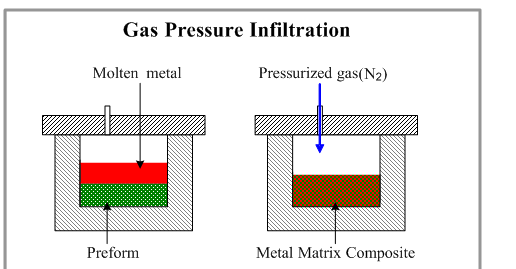
For large composite parts, the Gas Pressure Infiltration technique is used.
Due to the limited contact period of the fibres with the hot metal, the approach allows for the use of non-coated fibres.
In comparison to mechanical force approaches, Gas Pressure Infiltration causes less fibre damage.
2.9.1 Deposition
Vapor deposition encompasses a variety of production techniques involving the vaporization of a solid in a high-vacuum environment and the resulting vapor being deposited onto a target substrate. Capable of applying a coating at the single-atom or single-molecule level, vapor deposition techniques can create very pure, high-performance films of material.
The two main categories of vapor deposition are physical vapor deposition (PVD) and chemical vapor deposition (CVD).
2.9.2 Physical Vapor Deposition
While all PVD systems use the same basic principles, some of the techniques for producing and applying coating material differ.
Thermal evaporation and sputtering are the two most prevalent PVD procedures. The resulting vapour phase is then condensed onto the target substrate in both cases.
Thermal evaporation, one of the most fundamental PVD processes, entails heating a material in a vacuum chamber until the atoms on its exterior have enough energy to be liberated, a process known as vaporisation. The atoms are vaporised and then sent via a vacuum chamber to coat a target substrate above the source material.
Sputtering is a plasma-assisted technique that uses high-speed plasma ions to create vapour from a source material. The atoms, clusters of atoms, or molecules of the evaporated source material travel in a straight line. A "substrate" such as a silicon wafer will be covered by a thin coating of source material if it gets in the path of these streaming particles.
The atoms of the source material atomically connect to the substrate to form a thin layer. Sputtering can be done in a variety of ways, including diode, ion beam, and magnetron sputtering.
A target item can be spun on several axes or placed on a conveyor belt that runs through the plasma stream to form a consistent thin coating of a few atoms or molecules thick. With the same deposition method, single or multiple coatings can be applied.
Reactive gases such as oxygen or acetylene can also be used in the deposition chamber to create an extraordinarily strong bond between the coating and the substrate. Despite the fact that the thin films created by these techniques are only a few microns thick, they are incredibly robust, making PVD an excellent choice for a variety of applications.
2.9.3 Chemical Vapor Deposition
Chemical vapour deposition is a versatile and widely used method that may be tailored to a wide range of applications.
The employment of one or more chemical precursors that break down the source material and transport it to the substrate, where it is deposited, is the main distinction between PVD and CVD.
The substrate material is placed in a vacuum chamber, and the source material is placed either inside the same chamber or in an adjacent chamber in the typical CVD sequence. After that, the source material is heated or the atmospheric pressure is reduced until it vaporises. The source material is then exposed to one or more precursors, which react with it and allow it to be deposited on the substrate.
The vaporised substance reacts with the substrate to form a thin layer that is homogeneous in thickness. The thickness of the film can be controlled by changing the temperature and time of the process.
In recent years, the development of high-temperature CVD procedures has opened the door to a plethora of new commercial applications. For example, researchers have begun fabricating graphene sheets and large arrays of carbon nanotubes using high-temperature techniques, both of which have enormous potential for the construction of new electronics and other products.
One of the key advantages of CVD is that it can produce uniformly thick coatings even on complex structures. CVD, for example, can be used to coat carbon nanotubes with a consistent coating in order to change their mechanical properties, such as making them chemically respond in a specific way.
2.10.1 Interface Reactions of TiAl/TiSi2
As aforementioned, another excellent example for useful practice of diffusion pathway anaylsis is the Ti-Al-Si ternary system. The back scattered image of TiSi2/γ-TiAl annealed at 1,100 °C for 200 h is shown in Figure 10. Several phases including a porous layer developed at the interface of TiSi2 and TiAl. The phase sequence was obtained as TiAl/TiAl2/Ti2Al5/TiAl3 + Ti5Si4/Ti5Si4/TiSi/TiSi2. An irregular geometry of the Ti5Si4 phase was developed over a 100 μm layer thickness, in which the dark regions were observed as pores. It is noted that the Al solubility in the silicides (i.e., Ti5Si4 and TiSi) was detected to be less than 2 at %. However, the solubility of Si in the TiAl3 phase was detected to range up to 7 a t%, which is in agreement with previous results in which the large solubility of silicon in the TiAl3 phase has been reported.
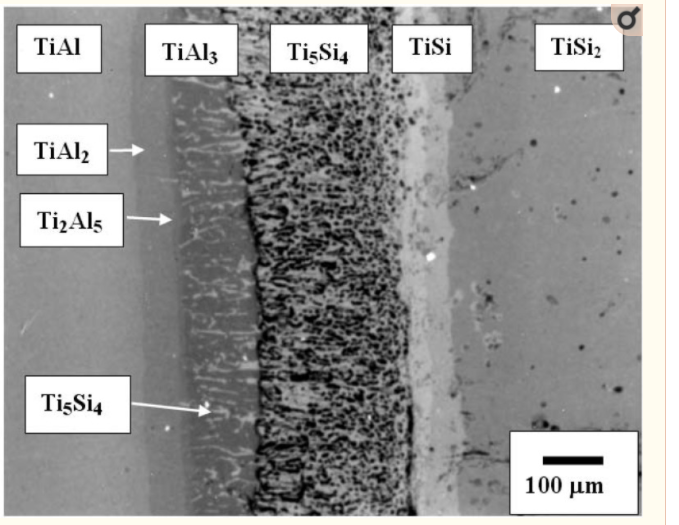
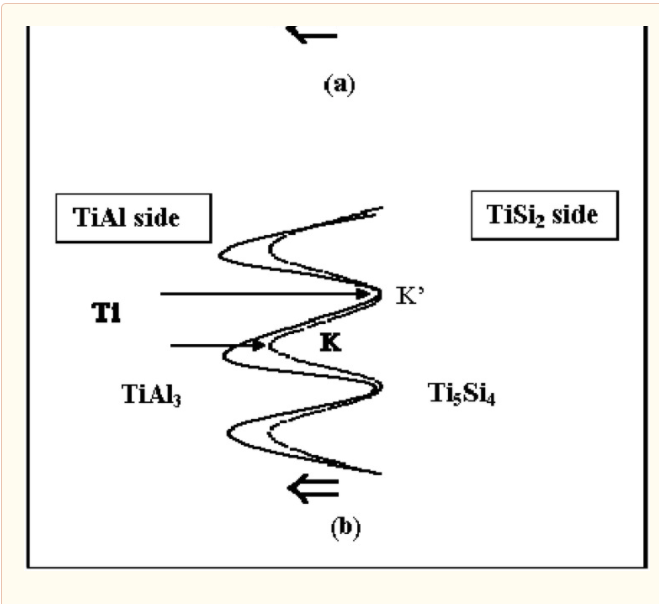
The information about partial kinetics can be obtained qualitatively from the formation of the columnar Ti5Si4 phase developed in the TiAl3 phase. In order to evaluate the kinetics of the movement of components from the resultant morphology, the rate limiting component has been evaluated. When initial driving force for an interdiffusion reaction is a composition difference, the Ti and Al atoms would move towards the TiSi2 side, but Si would diffuse in the direction of the TiAl side. The columnar zone of the Ti5Si4 phase grows towards the TiAl side as shown in Figure 11. If Si of the Ti5Si4 phase is the rate limiting component (D Ti(TiAl3) > D Si(Ti5Si4)), the feeding of Si atoms to the columnar Ti5Si4 phase determines the shape of the interface. Since the interface moves towards the TiAl side, the feeding rate to the point K’ is faster than that to the point K. Then, the pertubation would decay due to the faster movement of point, resulting that the planar interface develops. However, if Ti of the TiAl3 phase is the rate limiting component (D Ti(TiAl3) < D Si(Ti5Si4)), the arriving rate to the columnar Ti5Si4 phase establishes the shape of the interface. In this case, the arriving rate to the point K (the tip of the columnar Ti5Si4 phase) is faster than that to the point K’, resulting in the development of pertubations. Judging from the columnar morphology of the Ti5Si4 phase in Figure 10, the columnar Ti5Si4 phase grows through the TiAl3 phase, indicating that the rate limiting component is Titanium (D Ti(TiAl3)< D Si(Ti5Si4)).
The thickness () of the product phases was investigated as a function of the annealing time to determine the kinetic mode of each phase. The kinetics follow a parabolic law (2 = k t) when the intermediate phases increase through diffusion control. The thickness of the intermediate phases followed the parabolic law, according to the estimated data (Figure 12). The growth rate of Ti5Al4 was the most sensitive to the annealing period when compared to the growth rates of other phases. Despite the fact that the Ti5Si4 phase was developed as a porous structure, its growth rate is on par with that of the TiSi and TiAl3 phases, while the Ti2Al5 phase has the lowest growth rate of the product phases.
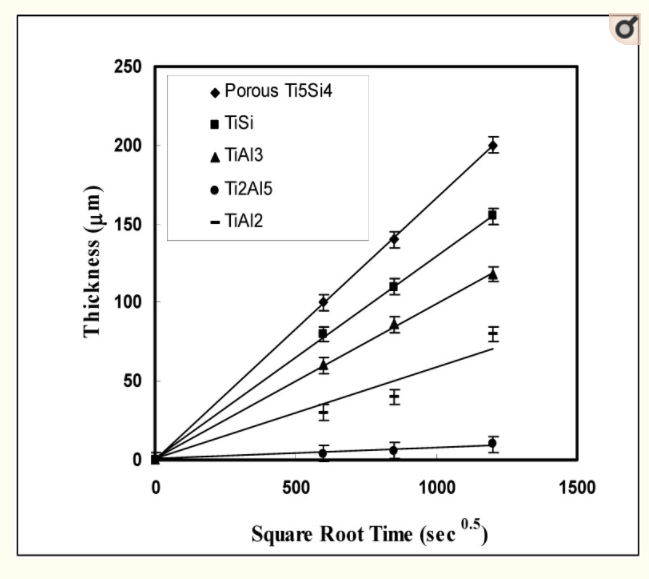
Since the titanium aluminide products show a limited Si solubility, it is useful to compare them with the growth in the binary system. It has been reported that most of the intermetallic phases such as Ti3Al, TiAl, TiAl2 and Ti2Al5 do not follow a parabolic law due to the influence of grain boundary diffusion with an exception of the TiAl3 phase which follows a parabolic law. However, Hirano and Iijima reported that as a result of diffusion annealing of β-Ti/TiAl couples, the Ti rich product phases (α-Ti, β-Ti and Ti3Al) are controlled by volume diffusion (following the parabolic law) at long annealing time (more than 200 h). Also, it has been pointed out that the reason for grain boundary diffusion in the Ti-Al system is due to the short annealing time. Therefore, based upon the above results, it is considered that when the reaction couples are examined at long term annealing, the parabolic growth mode is also dominant in the binary Ti-Al system. In this study, all of the product phases followed a parabolic law in agreement with the previous report.
The growth kinetics of the silicide formation also has been examined by Cockeram et al. The parabolic rate constants of TiSi and Ti5Si4 phase have been estimated as 1.19 × 10-10 cm2/sec and 5.44 × 10-11 cm2/sec, respectively, after Ti/Si diffusion annealing at 1,373 K. While it has been reported that the growth rate of TiSi was larger than that of Ti5Si4 , the current study shows that the growth rate of Ti5Si4 was larger than that of the TiSi phase. In fact, the estimated rate constants of the TiSi and Ti5Si4 phase were obtained as 1.58 × 10-10 cm2/sec and 2.67 × 10-10 cm2/sec respectively, indicating that the growth rate of Ti5Si4 has been increased about one order of magnitude higher than that of the previous report. It seems that this behavior is due to the effect of the third element (the effect of Al on the growth of Ti5Si4 phase).
In the reactive diffusion, the thicknesses of the product phases are related to the interdiffusion coefficients. However, when an intermetallic compound with a very narrow homogeneity range forms during reactive diffusion, the measurement of a concentration gradient is virtually not possible, because the concentration gradients of components does not develop clearly. In order to evaluate the diffusivity for an intermetallic compound having a limited solubility, Wagner introduced an integrated diffusion coefficient, Dint, which is defined as an integrated value of the interdiffusion coefficient
2.11.1 Metal matrix composites
Metal matrix composites (MMCs) are made up of metal matrix mixed with other metals, ceramics, and organic substances. Reinforcements are typically used to improve the base metal's various qualities. The particle dispersion has a significant impact on MMC characteristics. As a basis metal in metal matrix composites, copper, magnesium, and aluminium have gotten the greatest attention [1]. These MMCs are widely employed in a variety of areas, including aeroplanes, aerospace, autos, defence, and others [2]. Silicon Carbide (SiC), TiO2, Aluminium Oxide (Al2 O3), B4 C, Y2 O3, Si3 N4, and AlN are the most often utilised reinforcements [3-7]. The Al2 O3 reinforcement has a high compressive strength and is resistant to wear. Boron Carbide is one of the hardest elements known. It has a high elastic modulus and fracture toughness. Boron Carbide (B4 C) boosts the hardness of MMCs but does not improve their wear resistance considerably [4]. Fibers play a significant role as reinforcement because they impart strength to the matrix, allowing the physical and mechanical qualities to be improved as needed. For improved wear resistance, zircon is commonly utilised in hybrid reinforcement [5]. A lot of work has been done on fly ash reinforced MMCs in the recent decade. Because they are inexpensive and readily available as a waste by-product in thermal power plants. It enhances the MMCs' electromagnetic shielding effect [6-8]. This research explores the effect of varied dispersion on the mechanical characteristics of MMCs, processing techniques, and their applications, based on the claimed potential benefits of MMCs.
Many researchers have looked on MMCs that use a variety of metallic materials as a matrix. Al, Mg, Ti, Cu, and their alloys are the most attractive metals for industrial uses [8- 15]. Aluminium, both pure and alloyed, is the most studied material for use as a matrix in MMCs. Aluminum-based composites are excellent choices for structural use. As dispersion, several nano-sized oxides such as (Al2 O3, Y2 O3 ) [8-15], nitrides (Si3 N4, AlN) [12-14], carbides (TiC, SiC) [13-16], hydrates (TiH2 ) [7-9], and borides (TiB2) [8-13] were utilised. Ceramic reinforcing materials In these MMCs, carborundum, Sic, and alumina are commonly employed. Several researchers have also looked into other carbon allotropes such as carbon black, fullerenes, and carbon nanotubes [15-20]. CNTs are viable possibilities because they provide the metal matrix with extremely high mechanical characteristics. They also improve electrical conductivity, making MMCs a desirable material for electrical applications. MMCs utilise both single wall carbon nanotubes (SWCNT) and multi wall carbon nanotubes (MWCNT) as reinforcement. 0.1 wt. Percent copper MWCNT composites showed a 47 percent improvement in hardness and a bronze-0.1 percent increase in weight. Electrical conductivity was enhanced by 20% using SWCNT [18,19]. MMCs have also been found to disperse intermetallic complexes such as (NiAl, Al3 Ti) [15-19]. At high temperatures, Al– Al3Ti nanocomposite showed good mechanical properties, whereas TiAl–NiAl MMCs showed excellent hardness but poor fracture toughness.
And a poor socioeconomic standing (SES). In comparison to low SES children, high SES children have bigger median values for triceps skinfold, subscapular skinfold, arm circumference, and estimated mid-arm muscle and fat regions. High SES children have bigger values for all variables as compared to children in a US reference sample, while low SES children had smaller values. The disparities in arm fat area between the low SES children and the children in the other two groups, on the other hand, are bigger than the differences in arm muscle area. According to the findings, low-income Guatemalan children are more likely to suffer from chronic energy malnutrition than from protein malnutrition. For rural Guatemalan children, a similar pattern of energy deficiency has been seen. These findings show that estimates of arm fat reserves can be used to determine nutritional status in Third-World children. Muscle reserves are better than fat reserves as measures of nutritional status in underdeveloped nations, according to findings from rural Costa Rican and Honduran studies. However, those studies only looked at the extremes of the population distribution for muscle and fat and did not quantify cross-sectional muscle and fat areas.
2.11.2 Properties
Due to the limited wettability of ceramic nano-particles, traditional casting procedures for MMnCs result in an inhomogeneous dispersion of particles inside the matrix. The fundamental challenge in large-scale production of metal matrix nanocomposites is the limited wettability of ceramic nanoparticles, which prevents MMCs from being prepared using traditional casting procedures because the result would be an inhomogeneous distribution of particles within the matrix. The high surface energy causes agglomeration of nanoparticles, which are ineffective at preventing dislocation movement and can easily form a physical-chemical link with the matrix, lowering the nanoparticles' strength dramatically. Researchers have used a variety of unusual prepatration ways to tackle the wettability issue, such as in situ reinforcement creation or ex situ addition of ceramic reinforcement using particular methodologies.
2.12.1 Mechanical properties
We should presumably begin by acknowledging that the list of mechanical qualities is somewhat extensive. When characterising a substance, some are more vital and common than others. As a result, we're approaching the subject from the standpoint of an engineer. In order to make an informed judgement when designing something, someone needs to understand the fundamentals of metals.
2.12.1 Elastic properties
Plasticity is a mechanical feature of materials that demonstrates their ability to deform under tension without breaking and to keep the deformed shape after the load has been lifted. Forming is easier with metals that have a higher plasticity. Metal bending exemplifies this.
Ductility and malleability are two mechanical properties of materials that are related. Plasticity and ductility are two terms that describe a material's capacity to endure plastic deformation before breaking. It's measured in percentages of elongation or area decrease. Essentially, ductility is a quality that is required while drawing thin metal wires. Copper is an excellent example of a ductile material. This allows for the production of wires.
By definition, malleability is also comparable. However, it is used to describe a material's suitability for compressive deformation. In essence, a metal with good malleability is suitable for rolling or pounding metal plates or sheets.
2.13.1 What Is Ductility?
Ductility is a material's physical feature that allows it to be hammered thin or stretched into wire without breaking. A wire can be formed from a ductile materials.
Examples: Most metals, including gold, silver, copper, erbium, terbium, and samarium, are ductile materials. Tungsten and high-carbon steel are two examples of metals that aren't extremely ductile. In general, nonmetals are not ductile.
2.13.2 Room temperature strength
Intermetallics' chemical ordering reduces atomic mobility, resulting in enhanced resistance to plastic deformation at high temperatures. The inherent brittleness of polycrystalline ordered intermetallics at room temperature is due to this intrinsic source of high temperature strength. The needs for maximum high-temperature strength and ductility at room temperature are frequently irreconcilable. Up to 873 K, iron aluminides have a high strength. The yield and flow strengths have an unusual (positive) temperature dependence. Iron aluminides have yet to demonstrate adequate load bearing capabilities at higher temperatures. Alloy additions can improve both higher temperature strength and room temperature ductility, depending on which is more important for the application. Due to solid solution strengthening, elements like Cr, Ti, Mn, Co, and Mo cause increased flow stress. Elements like Zr, Ta, Nb, Re, and Hf partially dissolve in solution, reprecipitate, and effectively pin dislocations, causing strengthening. At high temperatures, Mo, Zr, and Hf have good tensile strength, but their ductility declines. Grain boundary cohesion strengthens Element B. Modification of the crystal structure by stoichiometry modifications, macroalloying, microalloying, and environmental control can all help increase room temperature ductility. B, TiB2, and Cr are known to improve ductility. In this regard, the study provides an overview of the current state of iron aluminides.
2.14.1 Thermal Properties of Concrete at Elevated Temperatures
Thermal conductivity, specific heat (or heat capacity), and mass loss are thermal parameters that influence temperature dependent qualities of concrete constructions. The aggregate type, moisture content, and composition of the concrete mix all have an impact on these qualities. Several test protocols have been developed to characterise the thermal characteristics of concrete at higher temperatures. Khaliq, Kodur et al., and Flynn provide a comprehensive assessment of the effect of temperature on the thermal characteristics of various concrete kinds.
2.14.2 Thermal Conductivity
The thermal conductivity of concrete changes with temperature and is between 1.4 and 3.6 W/m°K at room temperature [18]. Based on published test results and empirical relationships, Figure 1 depicts the fluctuation of NSC's thermal conductivity as a function of temperature. Khaliq [45] gathered the test data from many sources using experimental data [16, 20, 21, 24, 44, 48] and empirical relationships in several standards [4, 15]. The shaded area in Figure 1 depicts the variation in measured test results; this difference in reported data on thermal conductivity is mostly due to moisture content, aggregate type, test conditions, and measurement methodologies utilised in experiments [15, 18–20, 41]. It's worth noting that there aren't many established procedures for determining thermal characteristics. The maximum and lower bound values of thermal conductivity as defined by EC2 are also presented in Figure 1, and this range applies to all aggregate types. However, according to ASCE relations, the thermal conductivity illustrated in Figure 1 is appropriate to carbonate aggregates concrete.
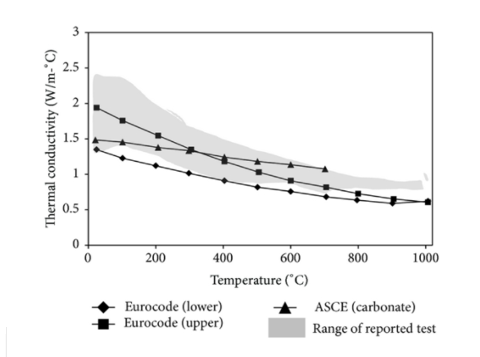
Overall thermal conductivity decreases with temperature, and this decline is influenced by the qualities of the concrete mix, particularly moisture content and permeability. This decrease in thermal conductivity can be linked to changes in moisture content as temperature rises.
Because of the low w/c ratio and the use of various binders in HSC, its thermal conductivity is higher than that of NSC [49]. At room temperature, the thermal conductivity of HSC is typically between 2.4 and 3.6 W/m°K. Fiber-reinforced concretes (with both steel and polypropylene fibres) have thermal conductivity that is practically same to ordinary concrete and is closer to HSC. As a result, no substantial effect of fibres on concrete thermal conductivity in the 20–800°C temperature range can be deduced.
2.14.3 Specific Heat
For different aggregate types, the specific heat of concrete at room temperature varies between 840 J/kgK and 1800 J/kgK. Specific heat is sometimes expressed in terms of thermal capacity, which is equal to the product of specific heat and concrete density. The specific heat property of concrete is affected by a variety of physical and chemical processes that occur at high temperatures. This includes the vaporization of free water at about 100°C, the dissociation of Ca(OH)2 into CaO and H2O between 400–500°C, and the quartz transformation of some aggregates above 600°C [24]. As a result, specific heat is strongly reliant on moisture content, and it rises dramatically as the water-to-cement ratio rises.
Khaliq and Kodur [27] collated data on concrete specific heat from a variety of research [16, 20, 24, 41, 44, 48]. Figure 2 shows how the specific heat of NSC varies with temperature, as reported in numerous studies based on test results and different standards. Up to 400°C, the specific heat of concrete type remains nearly constant, then increases to around 700°C, then remains constant between 700 and 800°C. The aggregate type has a major impact on the specific heat (thermal capacity) of concrete among the many components. The ASCE specified concrete specific heat relations [15] capture this effect. Carbonate aggregate concrete has higher specific heat (heat capacity) in 600–800°C temperature range and this is caused by an endothermic reaction, which results from decomposition of dolomite and absorbs a large amount of energy [12]. Carbonate aggregate concrete has a high heat capacity, which helps to reduce spalling and improve structural member fire resistance.
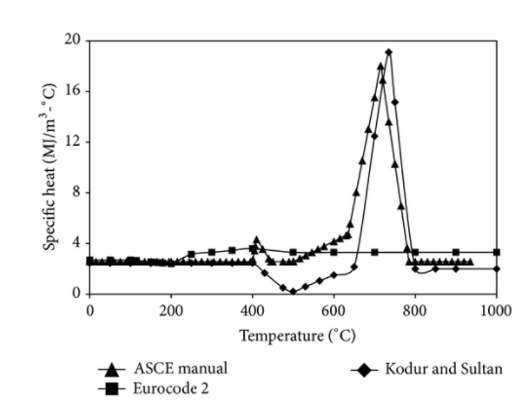
HSC has a slightly lower specific heat than NSC over the entire temperature range of 20–800°C [41]. The presence of fibres has a modest impact on the concrete's specific heat. The burning of polypropylene fibres in concrete provides microchannels for vapour release, resulting in a lower amount of heat absorbed for dehydration of chemically linked water, and hence a lower specific heat in the temperature range of 600–800°C. In the 400–800°C temperature range, however, concrete with steel fibres has a higher specific heat, which can be attributed to the increased heat consumed for dehydration of chemically linked water.
2.14.4 Mass Loss
Concretes are commonly categorised into two types based on density: (1) normal-weight concretes with densities ranging from 2150 to 2450 kgm3; and (2) lightweight concretes with densities ranging from 1350 to 1850 kgm3. Due to moisture loss, the density or mass of concrete drops as the temperature rises. The type of aggregate used in concrete has a significant impact on its retention in mass at high temperatures [21, 44].
Figure 3 depicts the variation in concrete mass as a function of temperature for carbonate and siliceous aggregate concretes. Up to 600°C, both carbonate and siliceous aggregate concretes lose very little mass. However, beyond 600°C, the kind of aggregate has a major impact on mass loss in concrete. Even at temperatures beyond 600°C, mass loss in siliceous aggregate concrete is negligible. Carbonate aggregate concrete, on the other hand, loses a greater percentage of its mass when heated above 600°C than siliceous aggregate concrete. The dissociation of dolomite in carbonate aggregate at roughly 600°C is responsible for the increased percentage of mass loss in carbonate aggregate concrete.
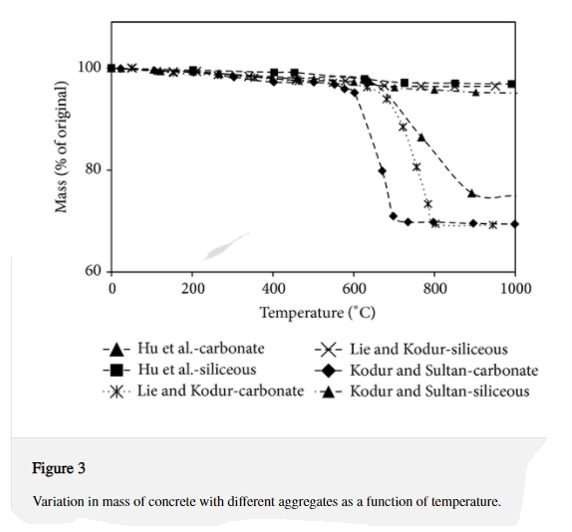
Because the strength of concrete has no effect on mass loss, HSC has a comparable mass loss pattern as NSC. Fiber-reinforced concrete loses mass at a similar rate to conventional concrete at temperatures of up to 800°C. Steel fiber-reinforced HSC has a somewhat lower mass loss than plain HSC above 800°C.
2.15.1 Fatigue resistance
Fatigue resistance is a measure of a bearing's strength, particularly in combustion engines, when the loading is cyclic in both direction and intensity. Fatigue spalling is a type of bearing failure that occurs when a subsurface stress field causes the bearing to fail in the depths of the material, close to the contacting surface (Gohar and Rahnejat, 2008). It can spread (proliferate) to the surface as a micro-spall, fracture, or pit. High contact pressures acting in compression can stop localised inelastic subsurface deformations like fractures. However, because the generated pressures in journal bearings are typically low to medium, minor cracks on the bearing surface can easily spread throughout the bearing material. When cracks interact and the crack geometry surrounds a newly isolated portion of bearing material, the bearing material is likely to break apart and disrupt the oil film in the region with the highest load and thinnest oil film thickness. Due to the mechanisms outlined under contamination, this would result in bearing seizure (see above). Furthermore, when pressure is applied to the detached piece of material, it is more likely to bond to the bearing or shaft.
Key takeaways:
- By minimising stress concentration on the weld joint, softening the weld toe shape, enhancing the metal structure of the weld zone, and altering the pin form and treatment conditions of friction stir welding, fatigue life can be extended.
- Alarm, resistance, and tiredness are the three stages. Alarm — This happens when we first perceive something as stressful, and our bodies respond by triggering the fight-or-flight reaction (as discussed earlier).
LTS stands for "low temperature superconductor," which refers to Nb-based alloys (most notably Nb-47wt% Ti) and A15 (Nb3Sn and Nb3Al) superconductors that were already in use before the discovery of "high temperature" copper-oxide superconductors in 1986. The temperature below which a superconductor must be chilled to become superconducting is referred to as "temperature" in this context; for LTS superconductors, this temperature is often much below 20 K (-253°C).
Because it can be made in a ductile form with the required nanostructure for high critical current density caused by strong vortex pinning, the Nb-47wt% Ti alloy has become the main commercial superconductor. Similarly, despite having a brittle A15 intermetallic phase, Nb3Sn can be made into robust composites with fine-grain nanostructures and high critical current densities in km lengths. Because they account for more than 95 percent of all superconducting magnets, Nb-Ti and Nb3Sn are often referred to as "technical superconductors." As in the case of the Large Hadron Collider (LHC) and typical Nb-based Superconducting Radio Frequency Cavity (SRF) applications, they normally require cooling to 4.2 K (liquid He is the most frequent coolant) and in some cases to below 2 K (superfluid liquid He). He gas is occasionally used at greater temperatures up to roughly 6 K.
Since the late 1970s, the Applied Superconductivity Center (ASC) has focused on understanding and enhancing these conductors for High Energy Physics and Fusion applications, but our breakthroughs have benefitted a wide range of LTS superconductor uses, including MRI and NMR devices.
The integration of processing, manufacturing microstructure, and comprehensive superconducting property characterization has been a significant element of the work at ASC. The ASC features a large facility for measuring superconducting properties, which is integrated with the center's own metallographic laboratory and the Magnet Lab's state-of-the-art equipments. The centre also boasts a hydrostatic extrusion press and a unique industrial quality wire production facility. The consequence has been a steady stream of seminal publications that have contributed to the massive improvement in LTS properties over the last 30 years.
The ductility and strength of extruded SiC-p/aluminum-alloy composites were examined by Cocen and Onel. In this study, Al-5Si-0.2Mg was melt stirred with 9, 13, 17, 22, and 26 vol. Percent SiC-p (15-30m) and extruded at 500°C with a 10:1 extrusion ratio. The use of extrusion allows the clusters of SiC particles to vanish, the porosity of the composite to be reduced to extremely low levels, and the yield and tensile strength values to be enhanced by around 40%. The ductility of the as-cast composites decreases as the amount of SiC-p increases, however extrusion results in a significant improvement in ductility due to the reduction in porosity. They discovered that the yield strength and tensile strength of as-cast composites rise with the volume percent SiC-p up to 17%, then decrease with further reinforcement additions. The yield and tensile strength of an extruded composite rise in lockstep with the volume fraction of reinforcement. Sahin researched the preparation of SiC-p reinforced AMMCs as well as their mechanical properties. He used the squeeze casting technique to create composites reinforced with SiC-p (10 and 20 wt. Percent and sizes of 29m, 45m, and 110m). They looked at how density, porosity, and hardness were affected. Before removing the Al-MMCs from the mould, 3000kg of force was applied mechanically for 7 minutes to reduce porosity. After that, the mould was removed from the press and allowed to cool for around 20 minutes. He discovered that while density fell as particle size fell, porosity and hardness reduced significantly as particle size increased. When the weight percent of reinforcement in a composite increases, the hardness, porosity, and density of the composite rise. When compared to other particle sizes (45m and 29m) reinforced composites, the composite with a particle size of 110m achieves a homogeneous distribution of SiC particles; nevertheless, when the particle size is less than 110m, some agglomeration occurs. El-Galy et al. Produced and characterised functionally graded centrifugal cast Al-SiC-p MMCs. SiC-p with varying weight fractions (0, 2.5, 5, 7.5, 10, and 15%) and three different particle sizes of 16m, 23m, and 500m has been examined. For feeding the metal along the tube axis, three rotational rates of 800rpm, 900rpm, or 1000rpm were employed, as well as two controlled linear speeds of 16mm/s or 28mm/s. The concentrations and hardness of SiC particles in the outside zone of the cast tubes attain their greatest value, followed by a gradual reduction in concentrations and hardness in the direction of the inner diameter. The outer zone hardness increases proportionally as the weight fraction of SiC-p increases, although beyond 10 wt. Percent SiC-p, the rate of rise reduces significantly. Tensile strength increases as the weight fraction of SiC-p increases, while ductility diminishes. They discovered that ultimate tensile strength is proportional to the fraction of SiC-p in the particles and inversely proportional to particle size. The tensile strength increases linearly up to 10 wt. Percent SiCp, then decreases until it reaches 15 wt. Percent SiC-p. Venkataraman and Sundararajan investigated the tensile strength of SiC-p reinforced Al MMCs and discovered that when the volume percentage of SiC in the Al matrix increases, the tensile strength increases but the tensile density drops. Ozben et al. Investigated mechanical and machinability parameters such as density, hardness, and impact resistance. 10 ISSN: 2456-7108 ISSN: 2456-7108 ISSN: 2456-7108 Patel et al., Adv. J. Grad. Res., Vol. 5, Issue 1, pp: 8-15, January 2019; available online at Journals.aijr.in Patel et al., Adv. J. Grad. Res., Vol. 5, Issue 1, pp: 8-15, January 2019 AlSi7Mg2 reinforced with 5, 10, and 15% wt.% SiC-p (30-60 m) was tested for toughness, tensile strength, and machinability. To improve the wettability of reinforcements, 2% Mg is added to Al7Si alloy. The ratio of 50–60 m particles remained lower than the ratio of 30-45 m particles in the particle size range (30-60 m). The use of varied sized particles is justified since small particles in a higher ratio aid in increasing strength while larger particles aid in achieving a homogeneous mixture. Hardness and density of MMCs rose with higher reinforcement ratio, while impact toughness dropped, according to their research. They also discovered that tensile strength rose up to 10% SiC-p reinforced and dropped when 15 wt.% SiC-p reinforcement was utilised. Surface roughness and cutting tool wear increase as the feed rate or particle ratio is raised. When the cutting speed of AlSi7Mg2-MMC samples was reduced, the surface quality increased. Rao et al. Conducted an experimental study on the mechanical properties of Al7075/SiC-p composites and discovered that increasing SiC particle size and weight percent significantly increased the tensile strength and hardness of the composites, but lowered the ductility. Ozden et al. [13] studied the impact behaviour of aluminium alloy (2124, 5083, and 6063 Al alloy) reinforced MMCs with hot extrusion ratios of 13.63:1 and 19.63:1 at different temperatures, i.e. -176 C, 21 C, 100 C, 200 C, and 300 C. They discovered that using SiCp as reinforcement in an Al alloy ductile matrix reduced the matrix's impact toughness. Increased particle size and hot extrusion ratio enhanced the impact toughness of the composites slightly, while artificial ageing reduced the impact toughness of all unreinforced alloys and composites. The impact behaviour of unreinforced AA5083 and AA5083-SiC-p composites was unaffected by the test temperature. The impact strength of composites based on AA2124 and AA6063 alloys decreases at 100 degrees Celsius. Meena et al. Investigated the mechanical properties of the created Al/SiC MMCs using a stir casting technique in which AA6063 was reinforced with SiC particulates (weight fractions of 5, 10, 15, and 20%) with mesh sizes of 220, 300, and 400, respectively. Proportionality limit, tensile strength upper yield point, tensile strength lower yield point, ultimate tensile strength, breaking strength, hardness, and density all increased as reinforced particulate size (220 mesh, 300 mesh, 400 mesh) and weight fraction (5 percent, 10%, 15%, and 20%) of SiC particles increased, but percent elongation and percent retraction decreased. Impact strength diminishes as the reinforced particulate size is larger, but increases as the weight percentage of SiC particles grows.
References:
1. Composite Materials: Engineering and Science, by Matthews and Rawlings, CRC Press.
2. An Introduction to composite material, by D.Hull and T.W. Clyne, Cambridge University press.
3. Metal Matrix Composites, Thermomechanical Behaviour by M.Taya, and R.J.Arsenault, Pergamon Press, Oxford.
4. Fundamentals of Metal Matrix Composites by S.Suresh, A.Martensen, and A.Needleman, Butterworth, Heinemann
5. Mechanics of composite materials, R. M. Jones, Mc Graw Hill Book Co.