Unit -X
Strategies for synthesis of organic compounds
A reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. It is quickly converted into the stable molecule when generated. The isolation and storage of these compounds is very exceptional case.
Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reaction in the next step is needed to destroy it.
When a reactive intermediate is not observable, its existence must be inferred through experimentation. This usually involves changing reaction conditions such as temperature or concentration and applying the techniques of chemical kinetics, chemical thermodynamics, or spectroscopy. We will often refer to certain reactive intermediates based on carbon, viz., carbocations, radicals, carbanions and carbenes.
Reactive intermediates have several features in common:
- Low concentration with respect to reaction substrate and final reaction product
- Often generated on chemical decomposition of a chemical compound
- It is often possible to prove the existence of this species by spectroscopic means
- Cage effects have to be taken into account
- Often stabilization by conjugation or resonance
- Often difficult to distinguish from a transition state
- Prove existence by means of chemical trapping
Elimination Reaction:
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Mechanism
The elimination reaction consists of three fundamental events they are:
- C-C pi bond is formed.
- Proton removal.
- There is a breakage in the bond of the leaving group.
Depending on the reaction kinetics, elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the molecularity.
E1 Reaction
- This is also called as unimolecular elimination reaction, there are usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
E2 Reaction:
- In an E2 mechanism which refers to bimolecular elimination is basically a one-step mechanism.
- Here, the carbon-hydrogen and carbon-halogen bonds mostly break off to form a new double bond.
- However, in the E2 mechanism, a base is part of the rate-determining step and it has a huge influence on the mechanism.
- The reaction rate is mostly proportional to the concentrations of both the eliminating agent and the substrate.
- It exhibits second-order kinetics.
The E2 mechanism can generally be represented as below. In the below-mentioned representation, B stands for base and X stands for the halogen.
Rearrangement:
The re-arrangement of carbon skeleton of a molecule to yield the structural isomers of the original molecule. Often a substituent moves form one atom to another aotm in the same molecule. The term “rearrangement” is used to describe two different types of organic chemical reactions. A rearrangement may involve the one step migration of an H atom or of a larger molecular fragment within a relatively short lived intermediate.
Curtius Rearrangement:
Curtius reaction involves the heating of an acyl azide which loses nitrogen and then rearranges to an isocyanate.
RCON3 → R-N=C=O + N2
If the reaction is performed in an alcoholic or aqueous medium, the isocyanate further reacts to form urethane, amine or substituted urea.
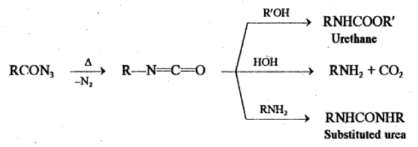

The conversion of acyl azides to isocyanates involves Curtius rearrangement whereas curtius reaction involves the conversion of acids to amines, urethane and substituted urea via Curtius rearrangement.
Acyl azide required for the reaction is obtained as follows.
RCOCl + NaN3 → RCON3 + NaCl
RCOOC2H5 → RCONHNH2 → RCON3 + 2H2O
Claisen Rearrangement:
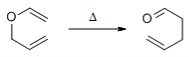
The aliphatic Claisen Rearrangement is a [3,3]-sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement is accompanied by a rearomatization:
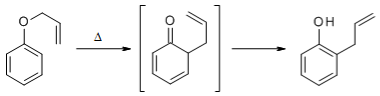
The etherification of alcohols or phenols and their subsequent Claisen Rearrangement under thermal conditions makes possible an extension of the carbon chain of the molecule.
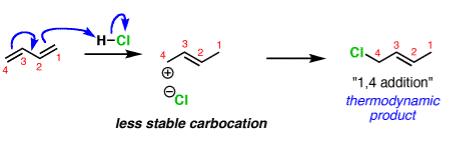
In organic chemistry the two different products can form depending on the reaction conditions. This is called kinetic and thermodynamic control.
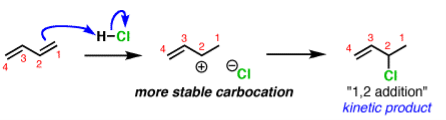
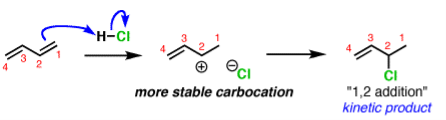
A common example is in additions of acids like HCl to dienes, such as butadiene.
The activation energy required to start the reaction. The activation energy is related to the stability of the carbocation that’s formed. Carbocations become more stabilized as the number of attached carbons increases. So the above example with a secondary carbocation is more stable than the bottom example primary carbocation is formed faster. This is the kinetic product.
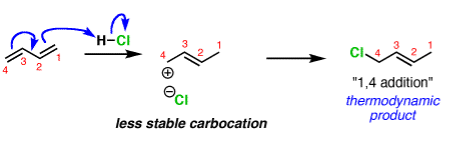
The reliability of the can opener is similar to the thermodynamic stability of the alkene that is formed. Alkenes increase in stability with the number of attached carbons. So the “1,4” product (where H and Cl add to C-1 and C-4 in our example) is more stable than the “1,2” product (where H and Cl add to C-1 and C-2) since the alkene has two carbon substituents. This is the thermodynamic product.
And money is analogous to the available energy (i.e. temperature). At low temperatures, when not much energy is available, the kinetic product will dominate. At high temperatures the thermodynamic product will be the major product.
The term “Solvents” refers to a class of chemical compounds described by function – the term derives from Latin, meaning roughly to “loosen.” In chemistry, solvents – which are generally in liquid form – are used to dissolve, suspend or extract other materials, usually without chemically changing either the solvents or the other materials.
Many different solvents are used in a wide variety of everyday product applications – from paint, personal care products and pharmaceuticals, to pesticides, cleaners and inks. Without solvents, many products we rely on would not perform as well.
Varied and versatile different solvents meet specific needs to make products with optimal performance attributes, including spray paints that dry quickly and don’t clog the spray nozzle, inks that don’t smudge, paints that look good and last a long time, and strong cleaners that are good for tough, greasy jobs.
Types of Solvents:
Hydrocarbon solvents are classified into three sub-groups based on the type of “carbon skeleton” of their molecules, giving us the aliphatic, aromatic and paraffinic solvents families. Paint thinner is a common example of a hydrocarbon solvent.
Oxygenated solvents are produced through chemical reactions from olefins, giving us the following sub-groups: alcohols, ketones, esters, ethers, glycol ethers and glycol ether esters. The human body naturally produces ketones when it burns fat.
Halogenated solvents are solvents that contain a halogen such as chlorine, bromine or iodine. Many people recognize perchloroethylene as an example – a highly effective solvent used in dry cleaning.
The role of solvents:
1) The solvents lead to the higher quality products.
2) The solvent selection enables a reduction in the number of synthesis steps.
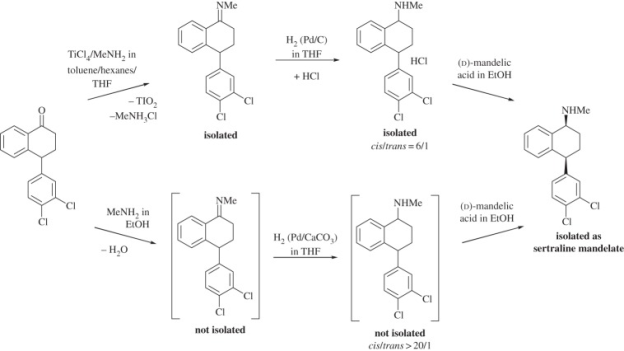
Pfizer sertaline synthesis
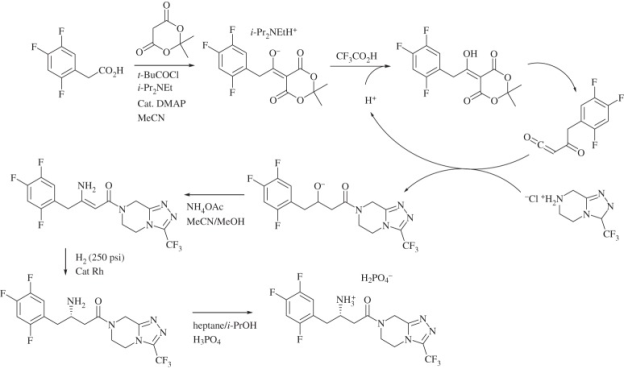
Mercks sitagliptin synthesis:
3) The solvent leads to a reduction of by-product formation.
4) The solvents enables product separation.
Unit -X
Strategies for synthesis of organic compounds
A reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. It is quickly converted into the stable molecule when generated. The isolation and storage of these compounds is very exceptional case.
Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reaction in the next step is needed to destroy it.
When a reactive intermediate is not observable, its existence must be inferred through experimentation. This usually involves changing reaction conditions such as temperature or concentration and applying the techniques of chemical kinetics, chemical thermodynamics, or spectroscopy. We will often refer to certain reactive intermediates based on carbon, viz., carbocations, radicals, carbanions and carbenes.
Reactive intermediates have several features in common:
- Low concentration with respect to reaction substrate and final reaction product
- Often generated on chemical decomposition of a chemical compound
- It is often possible to prove the existence of this species by spectroscopic means
- Cage effects have to be taken into account
- Often stabilization by conjugation or resonance
- Often difficult to distinguish from a transition state
- Prove existence by means of chemical trapping
Elimination Reaction:
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Mechanism
The elimination reaction consists of three fundamental events they are:
- C-C pi bond is formed.
- Proton removal.
- There is a breakage in the bond of the leaving group.
Depending on the reaction kinetics, elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the molecularity.
E1 Reaction
- This is also called as unimolecular elimination reaction, there are usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
E2 Reaction:
- In an E2 mechanism which refers to bimolecular elimination is basically a one-step mechanism.
- Here, the carbon-hydrogen and carbon-halogen bonds mostly break off to form a new double bond.
- However, in the E2 mechanism, a base is part of the rate-determining step and it has a huge influence on the mechanism.
- The reaction rate is mostly proportional to the concentrations of both the eliminating agent and the substrate.
- It exhibits second-order kinetics.
The E2 mechanism can generally be represented as below. In the below-mentioned representation, B stands for base and X stands for the halogen.
Rearrangement:
The re-arrangement of carbon skeleton of a molecule to yield the structural isomers of the original molecule. Often a substituent moves form one atom to another aotm in the same molecule. The term “rearrangement” is used to describe two different types of organic chemical reactions. A rearrangement may involve the one step migration of an H atom or of a larger molecular fragment within a relatively short lived intermediate.
Curtius Rearrangement:
Curtius reaction involves the heating of an acyl azide which loses nitrogen and then rearranges to an isocyanate.
RCON3 → R-N=C=O + N2
If the reaction is performed in an alcoholic or aqueous medium, the isocyanate further reacts to form urethane, amine or substituted urea.
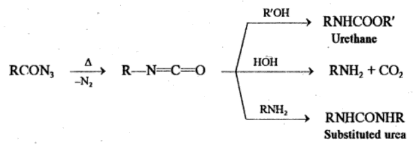

The conversion of acyl azides to isocyanates involves Curtius rearrangement whereas curtius reaction involves the conversion of acids to amines, urethane and substituted urea via Curtius rearrangement.
Acyl azide required for the reaction is obtained as follows.
RCOCl + NaN3 → RCON3 + NaCl
RCOOC2H5 → RCONHNH2 → RCON3 + 2H2O
Claisen Rearrangement:
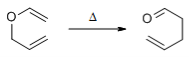
The aliphatic Claisen Rearrangement is a [3,3]-sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement is accompanied by a rearomatization:
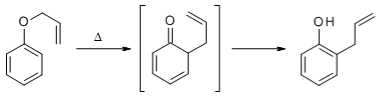
The etherification of alcohols or phenols and their subsequent Claisen Rearrangement under thermal conditions makes possible an extension of the carbon chain of the molecule.
In organic chemistry the two different products can form depending on the reaction conditions. This is called kinetic and thermodynamic control.
A common example is in additions of acids like HCl to dienes, such as butadiene.
The activation energy required to start the reaction. The activation energy is related to the stability of the carbocation that’s formed. Carbocations become more stabilized as the number of attached carbons increases. So the above example with a secondary carbocation is more stable than the bottom example primary carbocation is formed faster. This is the kinetic product.
The reliability of the can opener is similar to the thermodynamic stability of the alkene that is formed. Alkenes increase in stability with the number of attached carbons. So the “1,4” product (where H and Cl add to C-1 and C-4 in our example) is more stable than the “1,2” product (where H and Cl add to C-1 and C-2) since the alkene has two carbon substituents. This is the thermodynamic product.
And money is analogous to the available energy (i.e. temperature). At low temperatures, when not much energy is available, the kinetic product will dominate. At high temperatures the thermodynamic product will be the major product.
The term “Solvents” refers to a class of chemical compounds described by function – the term derives from Latin, meaning roughly to “loosen.” In chemistry, solvents – which are generally in liquid form – are used to dissolve, suspend or extract other materials, usually without chemically changing either the solvents or the other materials.
Many different solvents are used in a wide variety of everyday product applications – from paint, personal care products and pharmaceuticals, to pesticides, cleaners and inks. Without solvents, many products we rely on would not perform as well.
Varied and versatile different solvents meet specific needs to make products with optimal performance attributes, including spray paints that dry quickly and don’t clog the spray nozzle, inks that don’t smudge, paints that look good and last a long time, and strong cleaners that are good for tough, greasy jobs.
Types of Solvents:
Hydrocarbon solvents are classified into three sub-groups based on the type of “carbon skeleton” of their molecules, giving us the aliphatic, aromatic and paraffinic solvents families. Paint thinner is a common example of a hydrocarbon solvent.
Oxygenated solvents are produced through chemical reactions from olefins, giving us the following sub-groups: alcohols, ketones, esters, ethers, glycol ethers and glycol ether esters. The human body naturally produces ketones when it burns fat.
Halogenated solvents are solvents that contain a halogen such as chlorine, bromine or iodine. Many people recognize perchloroethylene as an example – a highly effective solvent used in dry cleaning.
The role of solvents:
1) The solvents lead to the higher quality products.
2) The solvent selection enables a reduction in the number of synthesis steps.
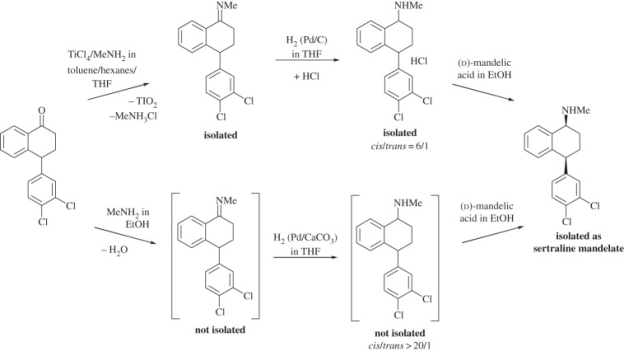
Pfizer sertaline synthesis
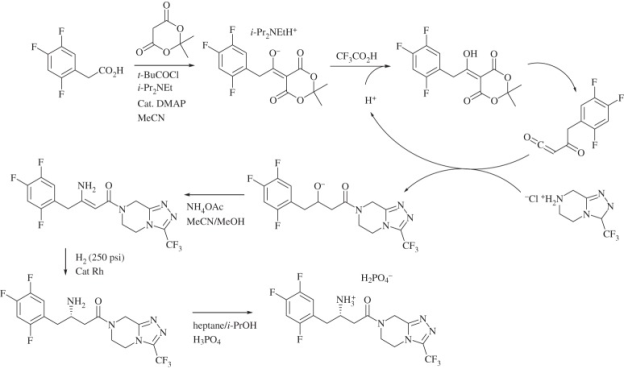
Mercks sitagliptin synthesis:
3) The solvent leads to a reduction of by-product formation.
4) The solvents enables product separation.