Unit – III
Use of free energy in Chemical Equilibria
Classical thermodynamics has a formal structure which serves to organize knowledge and to establish relationships between well–defined quantities. It is in this context that extensive observations are taken to imply that energy is conserved. Therefore, the change in the internal energy ∆U of a closed system is given by
∆U = q − w ……………………… (1)
Where q is the heat transferred into the system and w is the work done by the system. The historical sign convention is that heat added and work done by the system is positive, whereas heat given off and work done on the system are negative. Equation 1 may be written in differential form as
DU = dq – dw ……………………. (2)
For the special case where the system does work against a constant atmospheric pressure, this becomes
DU = dq − P dV ……………………… (3)
Where P is the pressure and V the volume. The specific heat capacity of a material is an indication of its ability to absorb or emit heat during a unit change in temperature. It is defined formally as dq/dT; since dq = dU + P dV, the specific heat capacity measured at constant volume is given by:
CV = µ ∂U ∂T ¶ V ………………(4)
It is convenient to define a new function H, the enthalpy of the system:
H = U + P V ………………….(5)
A change in enthalpy takes account of the heat absorbed at constant pressure, and the work done by the P ∆V term. The specific heat capacity measured at constant pressure is therefore given by:
CP = µ ∂H ∂T ¶ P
Entropy is used to describe the behavior of a system in terms of thermodynamic properties such as temperature, pressure, entropy, and heat capacity. This thermodynamic description took into consideration the state of equilibrium of the systems.
Meanwhile, the statistical definition which was developed at a later stage focused on the thermodynamic properties which were defined in terms of the statistics of the molecular motions of a system. Entropy is a measure of the molecular disorder.
In thermodynamics, energy-like property or state function of a system in thermodynamic equilibrium. Free energy has the dimensions of energy, and its value is determined by the state of the system and not by its history. Free energy is used to determine how systems change and how much work they can produce. It is expressed in two forms: the Helmholtz free energy F, sometimes called the work function, and the Gibbs free energy G. If U is the internal energy of a system, PV the pressure-volume product, and TS the temperature-entropy product (T being the temperature above absolute zero), then F = U − TS and G = U + PV − TS. The latter equation can also be written in the form G = H – TS, where H = U + PV is the enthalpy. Free energy is an extensive property, meaning that its magnitude depends on the amount of a substance in a given thermodynamic state.
Thermodynamic principles can be employed to derive a relation between electrical energy and the maximum amount of work (Wmx).
The maximum amount of work obtainable from the cell is the product of charge flowing per mole and maximum potential difference E, through which the charge is transferred.
Wmax=−nFE
where,
n= number of moles electrons transferred
F= Faraday (96495 coulombs)
E=emf of the cell
According to thermodynamics,
Wmax=ΔG
∴ΔG=−nFE
when E is positive, ΔG is positive, ΔG will be negative and the cell reaction is spontaneous.
This equation is named after the name of scientist who discovered is Walther Nernst. Nernst equation plays a major role in relating the Reduction Potential with the electrode potential, temperature of the chemicals which are undergoing the oxidation or reduction.(Reduction Potential is used to measure the tendency of the chemical species to acquire or loose electron to an electrode.)
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation ………..(i)
 G=-nFE ………..(ii)
G=-nFE ………..(ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE* …………. (iii)
G*=-nFE* …………. (iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ …………. (iv)
G= G*+RT lnQ …………. (iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ ……………. (v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6) ……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q ……. (vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q …….. (viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K ……. (ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Applications:
1- This is used in the solubility product and potentio-metric titration.
2- It is used to calculate the potential of ion charge.
3- It is used in oxygen and aquatic environment.
Corrosion: Electrochemical theory of corrosion: The destruction of metal by chemical or electrochemical attack of environment this process starting at the surface of metal known as corrosion. Corrosion is the two step process that requires three things i.e., a metallic surface, an electrolyte and oxygen. In the process of corrosion a metal atom at surface dissolve into an aqueous solution leaving the metal with excess negatively charged ions. These resultant ions are removed by a suitable electron acceptor. Corrosion can be thought of as the spontaneous return of metals to their ores through the process of oxidation.
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
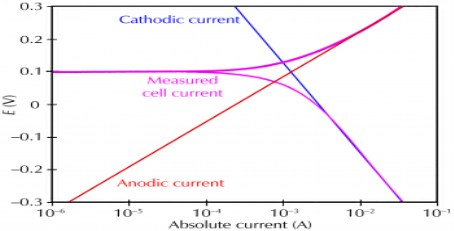
Corrosion process showing the anodic and cathodic component of current
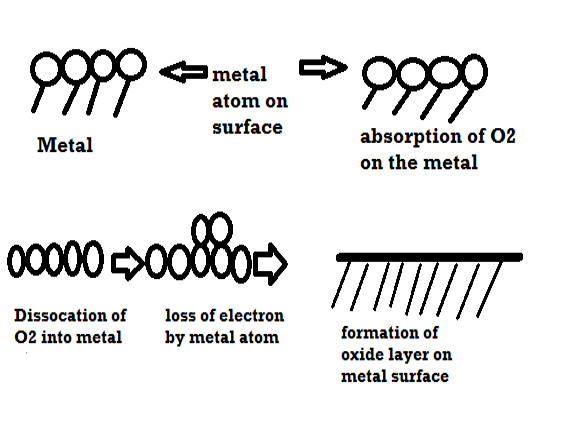
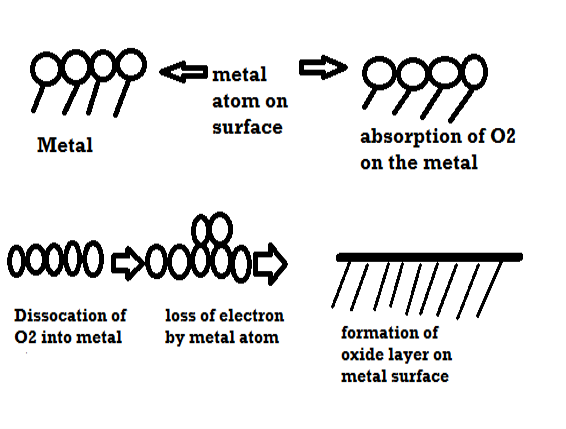
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection of metals having minimal tendency to interact galvanically, or will indicate the need or degree of protection to be applied to lessen the expected potential interactions. In general, the further apart the materials are in the galvanic series, the higher the risk of galvanic corrosion, which should be prevented by design. Conversely, the farther one metal is from another, the greater the corrosion will be. However, the series does not provide any information on the rate of galvanic corrosion and thus serves as a basic qualitative guide only.
Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
The uneven supply of oxygen to the same metal component leads to the formation of oxygen concentration cells that is called as the differential aeration theory of corrosion. It is the type of electrochemical corrosion that affects the metals such as steel and iron. The less oxygenated part behaves anodic while the more oxygenated part cathodic. Since cathodic reactions involve consumption of oxygen, the more oxygenated part behaves cathodic and less oxygenated pan behaves anodic. The reaction occurs because oppositely charged electrons flow between the smaller anode and larger cathode. Positively charged cations meeting negatively charged anions forming corrosion product and a resulting pit in the metal, otherwise known as pitting corrosion. In a gutter, pipe, tank or similar the anode is just below the waterline. This is where the oxidation occurs, corrosion product forms and a pit develops weakening the metal.
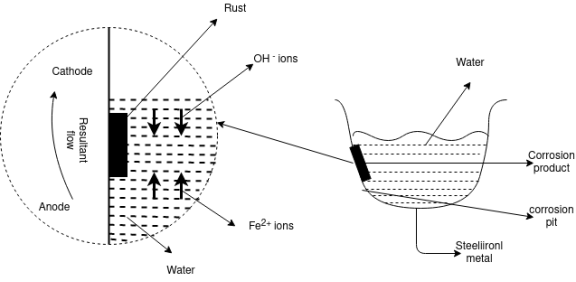
Waterline corrosion is a type of oxidation process that can happen to materials in contact with water. Waterline corrosion occurs when one portion of a base material is submersed in the water and another portion is in contact with the air. This creates a differential of the amount of oxygen in contact with the material's surface above and below the waterline and results in a corrosive reaction.
- It is a type of corrison caused by high concentration of sodium hydroxide in boiler water sodium carbonate hydroxide in the boiler and extent of hydrolysis increases with temprature.
Na2CO3 + H2O ----------------- >2Na.OH + CO2
2. Sodium hydroxide reacts with metal ion to form ion to form iron oxide and hydrogen.
3. When metral oxide coating cracks, chemical attack continues into metal along grain boundaries . Through the cracks , water flows , wwhen it evaporates sodium hydroxide is left there.
4. This attack iron of boiler.
5. This type of corrision of boiler parts particularly at stress parts caused due to chemical of caustic soda ( NaOH) Is called as embrittlement.
During water softening by soda – limes , processes Na2CO3 is added to water as precipitant . Caustic embrittlement can be prevented by addition lignins or tannos which help in blocking of hair cracks.
(i) Nature of metal
Position of metal in galvanic series.
If position is higher in galvanic series then it carrode faster
While for 2 metal the difference between them shows the corrosion ratio.
(ii) Potential Difference
If the difference at the electrode potential between two metal is high then the rate of corrosion would be also high while vice versa for lesser difference.
(iii) Purity of metal
Corrosion never took place in pure metals. While if metal itself has a impurity then galvanic cell set up easily which intend increases the rate of corrosion.
(iv) Relative areas of cathode and anode parts
Rate of corrosion is directly depends on the area of cathode and inversely depends on the area of anode. If the area of cathode is larger then there is more demand of electrons while in the smaller anode area the corrosion took place very fast.
(v) Nature of corrosion Product
Metal oxide film is formed on the surface of metal by corrosion due to oxygen. The formed film would be stable, unstable, volatile.
(vi) Temperature
At high temperature the rate of corrosion increases as because there is a consistent increase in the ionization and mobility difference rate while in some cases rate of corrosion decreases at high temperature as the solubility of O2 gas increases.
(vii) Presence of moisture
The rate of corrosion decreases in dry while increases in presence of moisture. Moisture act as the solvent for setting up of electrochemical corrosion.
(viii) Effect of pH
Rate of corrosion is high at acidic pH due to the evolution of H2 gas at cathode.
(ix) Concentration of electrolytes
This is also called as the Oxygen concentration cell. The rate of corrosion would be directly depend on the supply of oxygen on air.
(x) Over Voltage
The difference between the actual value and theoretical value of decomposition potential of electrode.
This is the technique used to control the corrosion on the surface of metal by formation of cathode layer on an electrochemical cell. There are 2 types of cathodic protections:
(i) Sacrificial Anodic Protection
(ii) Impressed Current Cathodic Protection
Unit – III
Use of free energy in Chemical Equilibria
Classical thermodynamics has a formal structure which serves to organize knowledge and to establish relationships between well–defined quantities. It is in this context that extensive observations are taken to imply that energy is conserved. Therefore, the change in the internal energy ∆U of a closed system is given by
∆U = q − w ……………………… (1)
Where q is the heat transferred into the system and w is the work done by the system. The historical sign convention is that heat added and work done by the system is positive, whereas heat given off and work done on the system are negative. Equation 1 may be written in differential form as
DU = dq – dw ……………………. (2)
For the special case where the system does work against a constant atmospheric pressure, this becomes
DU = dq − P dV ……………………… (3)
Where P is the pressure and V the volume. The specific heat capacity of a material is an indication of its ability to absorb or emit heat during a unit change in temperature. It is defined formally as dq/dT; since dq = dU + P dV, the specific heat capacity measured at constant volume is given by:
CV = µ ∂U ∂T ¶ V ………………(4)
It is convenient to define a new function H, the enthalpy of the system:
H = U + P V ………………….(5)
A change in enthalpy takes account of the heat absorbed at constant pressure, and the work done by the P ∆V term. The specific heat capacity measured at constant pressure is therefore given by:
CP = µ ∂H ∂T ¶ P
Entropy is used to describe the behavior of a system in terms of thermodynamic properties such as temperature, pressure, entropy, and heat capacity. This thermodynamic description took into consideration the state of equilibrium of the systems.
Meanwhile, the statistical definition which was developed at a later stage focused on the thermodynamic properties which were defined in terms of the statistics of the molecular motions of a system. Entropy is a measure of the molecular disorder.
In thermodynamics, energy-like property or state function of a system in thermodynamic equilibrium. Free energy has the dimensions of energy, and its value is determined by the state of the system and not by its history. Free energy is used to determine how systems change and how much work they can produce. It is expressed in two forms: the Helmholtz free energy F, sometimes called the work function, and the Gibbs free energy G. If U is the internal energy of a system, PV the pressure-volume product, and TS the temperature-entropy product (T being the temperature above absolute zero), then F = U − TS and G = U + PV − TS. The latter equation can also be written in the form G = H – TS, where H = U + PV is the enthalpy. Free energy is an extensive property, meaning that its magnitude depends on the amount of a substance in a given thermodynamic state.
Thermodynamic principles can be employed to derive a relation between electrical energy and the maximum amount of work (Wmx).
The maximum amount of work obtainable from the cell is the product of charge flowing per mole and maximum potential difference E, through which the charge is transferred.
Wmax=−nFE
where,
n= number of moles electrons transferred
F= Faraday (96495 coulombs)
E=emf of the cell
According to thermodynamics,
Wmax=ΔG
∴ΔG=−nFE
when E is positive, ΔG is positive, ΔG will be negative and the cell reaction is spontaneous.
This equation is named after the name of scientist who discovered is Walther Nernst. Nernst equation plays a major role in relating the Reduction Potential with the electrode potential, temperature of the chemicals which are undergoing the oxidation or reduction.(Reduction Potential is used to measure the tendency of the chemical species to acquire or loose electron to an electrode.)
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation ………..(i)
 G=-nFE ………..(ii)
G=-nFE ………..(ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE* …………. (iii)
G*=-nFE* …………. (iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ …………. (iv)
G= G*+RT lnQ …………. (iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ ……………. (v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6) ……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q ……. (vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q …….. (viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K ……. (ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Applications:
1- This is used in the solubility product and potentio-metric titration.
2- It is used to calculate the potential of ion charge.
3- It is used in oxygen and aquatic environment.
Corrosion: Electrochemical theory of corrosion: The destruction of metal by chemical or electrochemical attack of environment this process starting at the surface of metal known as corrosion. Corrosion is the two step process that requires three things i.e., a metallic surface, an electrolyte and oxygen. In the process of corrosion a metal atom at surface dissolve into an aqueous solution leaving the metal with excess negatively charged ions. These resultant ions are removed by a suitable electron acceptor. Corrosion can be thought of as the spontaneous return of metals to their ores through the process of oxidation.
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
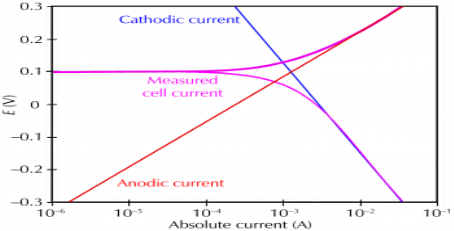
Corrosion process showing the anodic and cathodic component of current
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection of metals having minimal tendency to interact galvanically, or will indicate the need or degree of protection to be applied to lessen the expected potential interactions. In general, the further apart the materials are in the galvanic series, the higher the risk of galvanic corrosion, which should be prevented by design. Conversely, the farther one metal is from another, the greater the corrosion will be. However, the series does not provide any information on the rate of galvanic corrosion and thus serves as a basic qualitative guide only.
Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
The uneven supply of oxygen to the same metal component leads to the formation of oxygen concentration cells that is called as the differential aeration theory of corrosion. It is the type of electrochemical corrosion that affects the metals such as steel and iron. The less oxygenated part behaves anodic while the more oxygenated part cathodic. Since cathodic reactions involve consumption of oxygen, the more oxygenated part behaves cathodic and less oxygenated pan behaves anodic. The reaction occurs because oppositely charged electrons flow between the smaller anode and larger cathode. Positively charged cations meeting negatively charged anions forming corrosion product and a resulting pit in the metal, otherwise known as pitting corrosion. In a gutter, pipe, tank or similar the anode is just below the waterline. This is where the oxidation occurs, corrosion product forms and a pit develops weakening the metal.
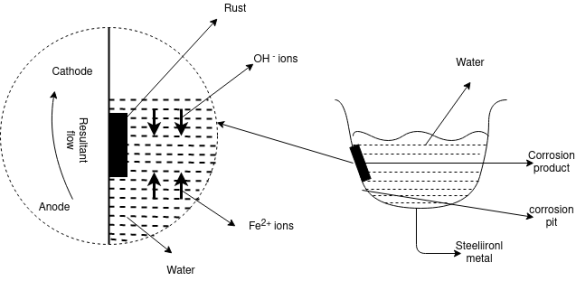
Waterline corrosion is a type of oxidation process that can happen to materials in contact with water. Waterline corrosion occurs when one portion of a base material is submersed in the water and another portion is in contact with the air. This creates a differential of the amount of oxygen in contact with the material's surface above and below the waterline and results in a corrosive reaction.
- It is a type of corrison caused by high concentration of sodium hydroxide in boiler water sodium carbonate hydroxide in the boiler and extent of hydrolysis increases with temprature.
Na2CO3 + H2O ----------------- >2Na.OH + CO2
2. Sodium hydroxide reacts with metal ion to form ion to form iron oxide and hydrogen.
3. When metral oxide coating cracks, chemical attack continues into metal along grain boundaries . Through the cracks , water flows , wwhen it evaporates sodium hydroxide is left there.
4. This attack iron of boiler.
5. This type of corrision of boiler parts particularly at stress parts caused due to chemical of caustic soda ( NaOH) Is called as embrittlement.
During water softening by soda – limes , processes Na2CO3 is added to water as precipitant . Caustic embrittlement can be prevented by addition lignins or tannos which help in blocking of hair cracks.
(i) Nature of metal
Position of metal in galvanic series.
If position is higher in galvanic series then it carrode faster
While for 2 metal the difference between them shows the corrosion ratio.
(ii) Potential Difference
If the difference at the electrode potential between two metal is high then the rate of corrosion would be also high while vice versa for lesser difference.
(iii) Purity of metal
Corrosion never took place in pure metals. While if metal itself has a impurity then galvanic cell set up easily which intend increases the rate of corrosion.
(iv) Relative areas of cathode and anode parts
Rate of corrosion is directly depends on the area of cathode and inversely depends on the area of anode. If the area of cathode is larger then there is more demand of electrons while in the smaller anode area the corrosion took place very fast.
(v) Nature of corrosion Product
Metal oxide film is formed on the surface of metal by corrosion due to oxygen. The formed film would be stable, unstable, volatile.
(vi) Temperature
At high temperature the rate of corrosion increases as because there is a consistent increase in the ionization and mobility difference rate while in some cases rate of corrosion decreases at high temperature as the solubility of O2 gas increases.
(vii) Presence of moisture
The rate of corrosion decreases in dry while increases in presence of moisture. Moisture act as the solvent for setting up of electrochemical corrosion.
(viii) Effect of pH
Rate of corrosion is high at acidic pH due to the evolution of H2 gas at cathode.
(ix) Concentration of electrolytes
This is also called as the Oxygen concentration cell. The rate of corrosion would be directly depend on the supply of oxygen on air.
(x) Over Voltage
The difference between the actual value and theoretical value of decomposition potential of electrode.
This is the technique used to control the corrosion on the surface of metal by formation of cathode layer on an electrochemical cell. There are 2 types of cathodic protections:
(i) Sacrificial Anodic Protection
(ii) Impressed Current Cathodic Protection