Unit -IX
Reactivity of organic molecules
The organic compound that possesses acidic properties is called as an organic acid. E.g.: carboxylic acid, whose acidity is associated with their carboxyl group –COOH. The relative stability of the conjugate base of the acid determines its acidity.
The organic compound that possesses basic properties is called as organic bases. Organic bases act as the protons acceptors. Organic bases usually contain the nitrogen atoms that can be easily protonated. E.g.: Amines that have a lone pair of electron on the nitrogen atom and hence it can act as the proton acceptor.
Bond Strength:
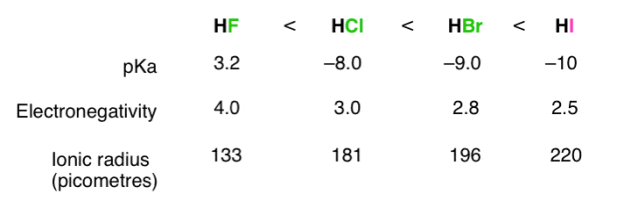
The stronger the A–H or B–H+ bond, the less likely the bond is to break to form H+H+ ions and thus the less acidic the substance. This effect can be illustrated using the hydrogen halides:
Acid Strength | HF | HCl | HBr | HI |
H-X Bond Energy | 570 | 432 | 366 | 298 |
PKa | 3.20 | -6.1 | -8.9 | -9.3 |
The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases.
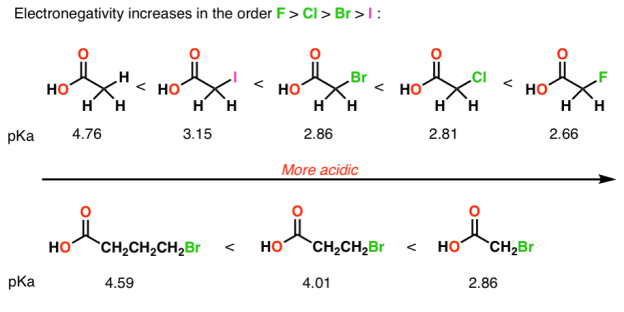
A negative charge prefers to rest on electronegative elements. Therefore, a negative charge is more stable on oxygen than it is on nitrogen similarly, a negative charge is more stable on nitrogen than it is on carbon. For that reason, alcohols (R—OH) are more acidic than amines (R—NH2), which in turn are more acidic than alkanes (R—CH3), as shown here:
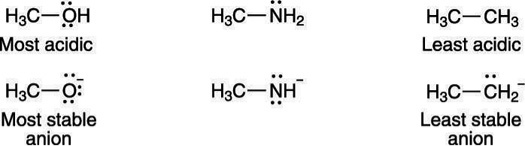
Electro negativity affects acid strength:
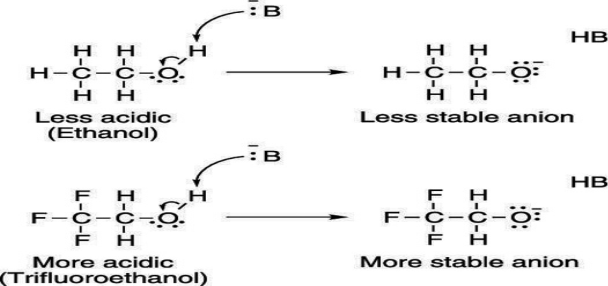
Electron-withdrawing groups on an acid also stabilize the conjugate base anion by allowing some of the charge on the anion to delocalize to other parts of the molecule. For example, trifluoroethanol (shown here) is more acidic than ethanol.
Bronsted-Lowry acid: The Bronsted-Lowry theory describes acid-base interactions in terms of proton transfer between chemical species. A Bronsted-Lowry acid is any species that can donate a proton, H+ and a base is any species that can accept a proton. In terms of chemical structure, this means that any Bronsted-Lowry acid must contain a hydrogen that can dissociate as H+. In order to accept a proton, a Bronsted-Lowry base must have at least one lone pair of electrons to form a new bond with a proton. Any species that is capable of donating a proton –H+ while the species that is capable of accepting a protons that requires a lone pair of electron to bond to the H+. E.g.: Water; as it is amphoteric that means it can act as both Bronsted-Lowry acid and a Bronsted-Lowry base. The ionization of weak acids and bases is partial while it is complete in strong acids and bases. The conjugate base of a Bronsted-Lowry acid is the species formed after an acid donates a proton. The conjugate acid of a Bronsted-Lowry base is the species formed after a base accepts a proton. The two species in a conjugate acid-base pair have the same molecular formula except the acid has an extra H+ ion compared to the conjugate base.
Factors influencing acidity:
1) Charge: The proton removal tends to decrease the formal charge on an atom by one unit.
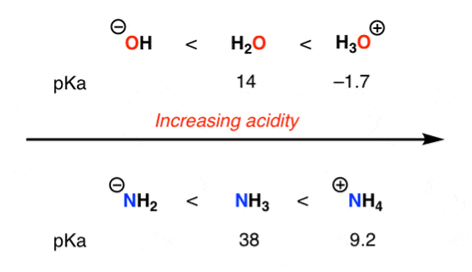
Acidity increases with increasing positive charge of an atom
2) The role of an atom: On going across a row in the periodic table the acidity increases. According to this HF is more electro negativity than H2O, NH3 and CH4 due to greater electro negativity of fluorine.
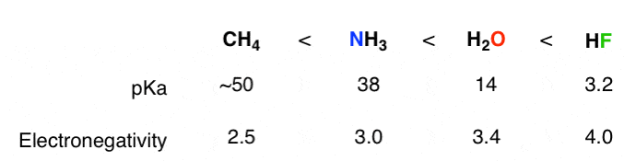
Across the periodic table acidity increases with electro negativity
 Acidity increases with electro negativity on moving across the periodic table
Acidity increases with electro negativity on moving across the periodic table
3) Resonance
A huge stabilizing factor for a conjugate base is if the negative charge can be delocalized through resonance. The classic examples are with phenol (C6H5OH) which is about a million times more acidic than water, and with acetic acid (pKa of ~5).
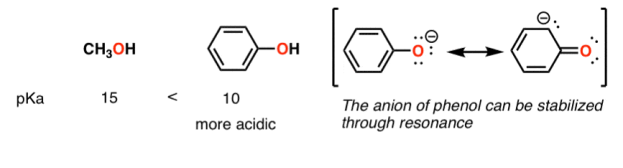
Contrast methanol with phenol
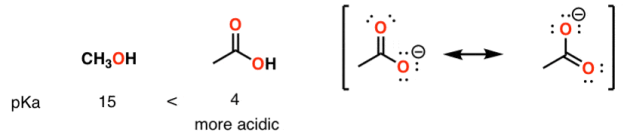
Alcohol vs carboxylic acid
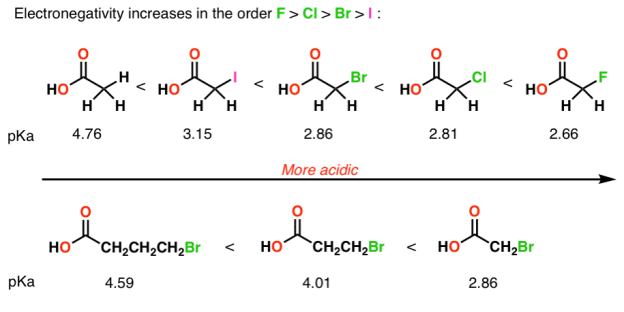
4) Inductive effects
Electronegative atoms can draw negative charge toward themselves, which can lead to considerable stabilization of conjugate bases.
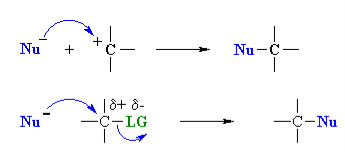
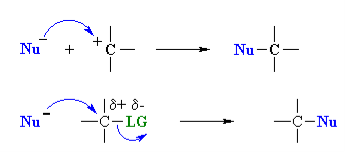
Nucleophilicity: The donation of electrons from one atom to another except to the hydrogen for the formation of bond is called as the nucleophile. The name nucleophile means nucleus loving and indicates that it attacks regions of low electron density in the substrate molecule. They may be negative ions including carbanions or neutral molecules with free electron pair. A nucleophile can be represented by Nu:-.
E.g: Cl-, Br-, CN- etc.
There are at least four key factors contributing to nucleophilicity.
- Charge
- Electronegativity
- Solvent
- Steric hindrance.
- A nucleophile is a species that donates a pair of electrons to form a new covalent bond. Nucleophilicity is measured by comparing reaction rates; the faster the reaction, the better (or, “stronger”) the nucleophile.
Measurement of Nucleophilicity:
They possess the properties roughly parallel to the basicity, beyond that they possess two additional factors:
Steric Hindrance Reaction where carbon is an electrophile are often more difficult than reactions where a proton is the electrophile because the orbitals involved are not as accessible. This is called as the steric hindrance and it can affect reaction of nucleophile with electrophile.
Solvation The medium in which a reaction takes place can greatly affect the rate of a reaction. Specially the solvent can greatly affect the nucleophilicity of a Lewis base through hydrogen-bonding interactions.
Basicity: The donation of electron pair form base to the protron. This occurs in the base moclecule in order to attain the stability.
E.g.:
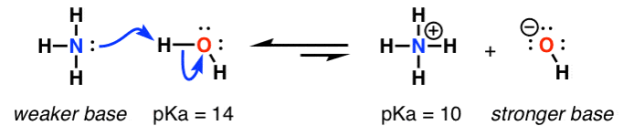
Measurement of basicity:
The most of the species participate in reversible acid-base reactions, the basicity can be measured by the position of an equilibrium.
Thermodynamic control of reaction: They are also called as the thermodynamic control products. Thermodynamic basically deals with the control of potential energy.
R A + B (more stable)
 On the condition when product B is more stable then G is taken in consideration. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favored under kinetic control and B is the thermodynamic product and is favored under thermodynamic control.
On the condition when product B is more stable then G is taken in consideration. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favored under kinetic control and B is the thermodynamic product and is favored under thermodynamic control.
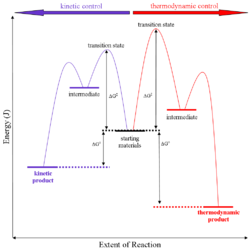
Kinetic control of reaction: They are also called as the kinetic control product. Kinetic control of reaction basically deals with the activation energy for the transition state.
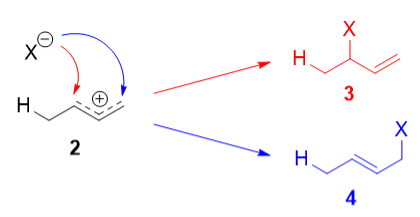
The reaction of one equivalent of hydrogen bromide with 1,3-butadiene gives different products at under different conditions and is a classic example of the concept of thermodynamic versus kinetic control of a reaction
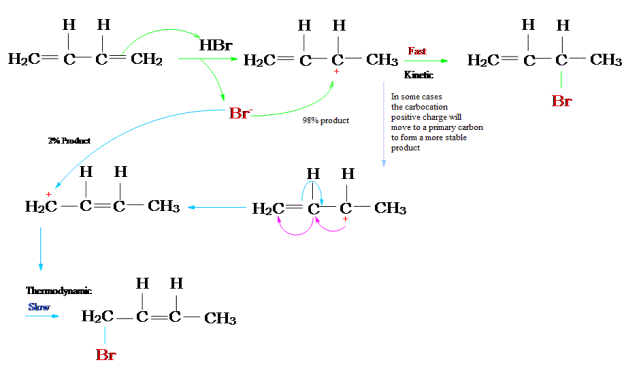
The above reaction can be termed in graphical representation
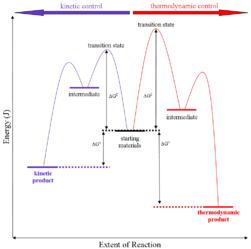
Mechanism:
Protonation of one of the C=C double bonds took place. Protonation took place to give more stable carbocation.
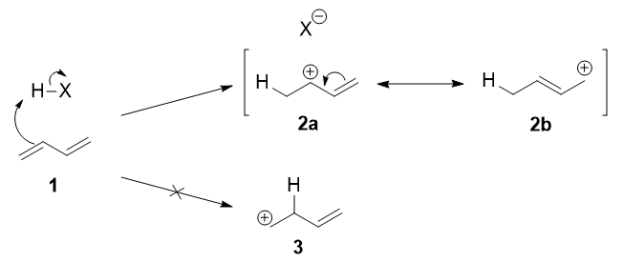
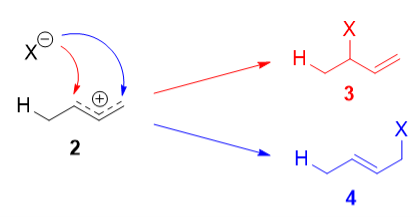
The more stable cation is not only secondary, but also allylic, and therefore enjoys stabilization via resonance. This is depicted in the resonance forms in 2a and 2b above. This allylic carbocation, more properly denoted as the resonance hybrid 2, has two carbons which have significant positive charge, and the bromide ion can attack either carbon. Attacking the central carbon, adjacent to the site of protonation, leads to the kinetic product 3 that is called as the 1,2-adduct; attacking the terminal carbon, distant from the site of protonation, leads to the thermodynamic product 4 that is called as the 1,4-adduct.
Unit -IX
Reactivity of organic molecules
The organic compound that possesses acidic properties is called as an organic acid. E.g.: carboxylic acid, whose acidity is associated with their carboxyl group –COOH. The relative stability of the conjugate base of the acid determines its acidity.
The organic compound that possesses basic properties is called as organic bases. Organic bases act as the protons acceptors. Organic bases usually contain the nitrogen atoms that can be easily protonated. E.g.: Amines that have a lone pair of electron on the nitrogen atom and hence it can act as the proton acceptor.
Bond Strength:
The stronger the A–H or B–H+ bond, the less likely the bond is to break to form H+H+ ions and thus the less acidic the substance. This effect can be illustrated using the hydrogen halides:
Acid Strength | HF | HCl | HBr | HI |
H-X Bond Energy | 570 | 432 | 366 | 298 |
PKa | 3.20 | -6.1 | -8.9 | -9.3 |
The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases.
A negative charge prefers to rest on electronegative elements. Therefore, a negative charge is more stable on oxygen than it is on nitrogen similarly, a negative charge is more stable on nitrogen than it is on carbon. For that reason, alcohols (R—OH) are more acidic than amines (R—NH2), which in turn are more acidic than alkanes (R—CH3), as shown here:
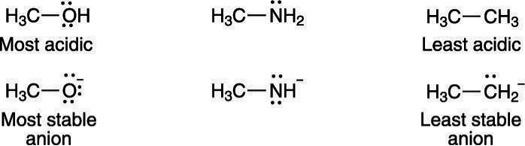
Electro negativity affects acid strength:
Electron-withdrawing groups on an acid also stabilize the conjugate base anion by allowing some of the charge on the anion to delocalize to other parts of the molecule. For example, trifluoroethanol (shown here) is more acidic than ethanol.
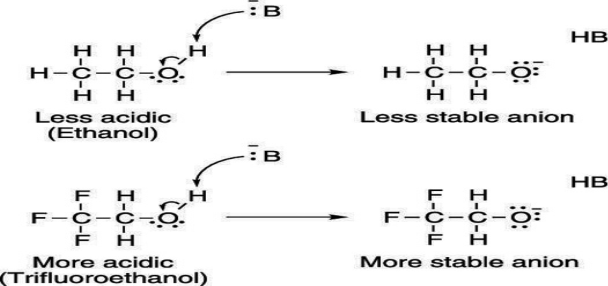
Bronsted-Lowry acid: The Bronsted-Lowry theory describes acid-base interactions in terms of proton transfer between chemical species. A Bronsted-Lowry acid is any species that can donate a proton, H+ and a base is any species that can accept a proton. In terms of chemical structure, this means that any Bronsted-Lowry acid must contain a hydrogen that can dissociate as H+. In order to accept a proton, a Bronsted-Lowry base must have at least one lone pair of electrons to form a new bond with a proton. Any species that is capable of donating a proton –H+ while the species that is capable of accepting a protons that requires a lone pair of electron to bond to the H+. E.g.: Water; as it is amphoteric that means it can act as both Bronsted-Lowry acid and a Bronsted-Lowry base. The ionization of weak acids and bases is partial while it is complete in strong acids and bases. The conjugate base of a Bronsted-Lowry acid is the species formed after an acid donates a proton. The conjugate acid of a Bronsted-Lowry base is the species formed after a base accepts a proton. The two species in a conjugate acid-base pair have the same molecular formula except the acid has an extra H+ ion compared to the conjugate base.
Factors influencing acidity:
1) Charge: The proton removal tends to decrease the formal charge on an atom by one unit.
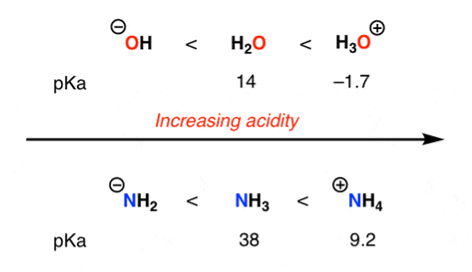
Acidity increases with increasing positive charge of an atom
2) The role of an atom: On going across a row in the periodic table the acidity increases. According to this HF is more electro negativity than H2O, NH3 and CH4 due to greater electro negativity of fluorine.
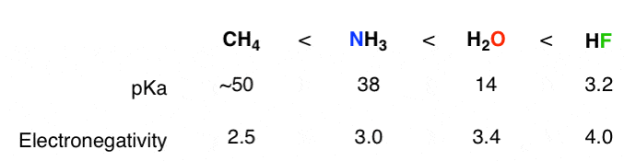
Across the periodic table acidity increases with electro negativity
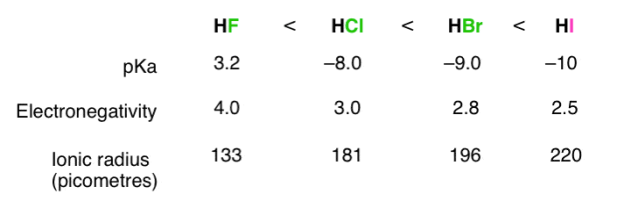 Acidity increases with electro negativity on moving across the periodic table
Acidity increases with electro negativity on moving across the periodic table
3) Resonance
A huge stabilizing factor for a conjugate base is if the negative charge can be delocalized through resonance. The classic examples are with phenol (C6H5OH) which is about a million times more acidic than water, and with acetic acid (pKa of ~5).
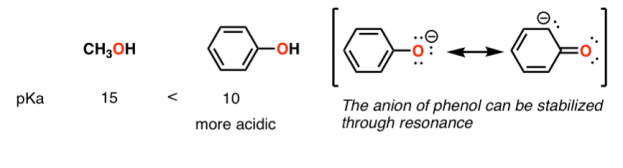
Contrast methanol with phenol
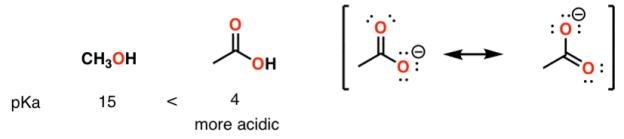
Alcohol vs carboxylic acid
4) Inductive effects
Electronegative atoms can draw negative charge toward themselves, which can lead to considerable stabilization of conjugate bases.
Nucleophilicity: The donation of electrons from one atom to another except to the hydrogen for the formation of bond is called as the nucleophile. The name nucleophile means nucleus loving and indicates that it attacks regions of low electron density in the substrate molecule. They may be negative ions including carbanions or neutral molecules with free electron pair. A nucleophile can be represented by Nu:-.
E.g: Cl-, Br-, CN- etc.
There are at least four key factors contributing to nucleophilicity.
- Charge
- Electronegativity
- Solvent
- Steric hindrance.
- A nucleophile is a species that donates a pair of electrons to form a new covalent bond. Nucleophilicity is measured by comparing reaction rates; the faster the reaction, the better (or, “stronger”) the nucleophile.
Measurement of Nucleophilicity:
They possess the properties roughly parallel to the basicity, beyond that they possess two additional factors:
Steric Hindrance Reaction where carbon is an electrophile are often more difficult than reactions where a proton is the electrophile because the orbitals involved are not as accessible. This is called as the steric hindrance and it can affect reaction of nucleophile with electrophile.
Solvation The medium in which a reaction takes place can greatly affect the rate of a reaction. Specially the solvent can greatly affect the nucleophilicity of a Lewis base through hydrogen-bonding interactions.
Basicity: The donation of electron pair form base to the protron. This occurs in the base moclecule in order to attain the stability.
E.g.:
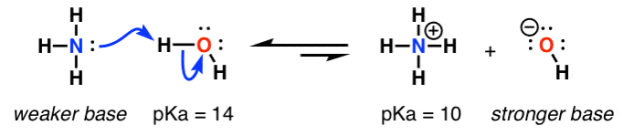
Measurement of basicity:
The most of the species participate in reversible acid-base reactions, the basicity can be measured by the position of an equilibrium.
Thermodynamic control of reaction: They are also called as the thermodynamic control products. Thermodynamic basically deals with the control of potential energy.
R A + B (more stable)
 On the condition when product B is more stable then G is taken in consideration. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favored under kinetic control and B is the thermodynamic product and is favored under thermodynamic control.
On the condition when product B is more stable then G is taken in consideration. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favored under kinetic control and B is the thermodynamic product and is favored under thermodynamic control.
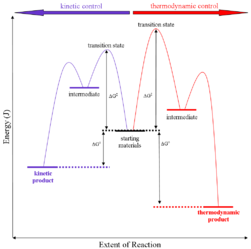
Kinetic control of reaction: They are also called as the kinetic control product. Kinetic control of reaction basically deals with the activation energy for the transition state.
The reaction of one equivalent of hydrogen bromide with 1,3-butadiene gives different products at under different conditions and is a classic example of the concept of thermodynamic versus kinetic control of a reaction
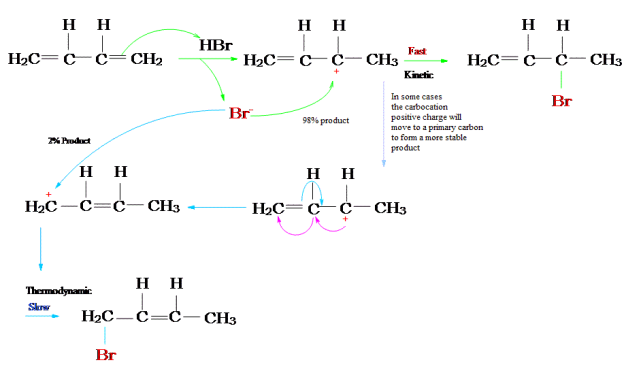
The above reaction can be termed in graphical representation
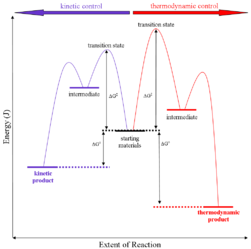
Mechanism:
Protonation of one of the C=C double bonds took place. Protonation took place to give more stable carbocation.
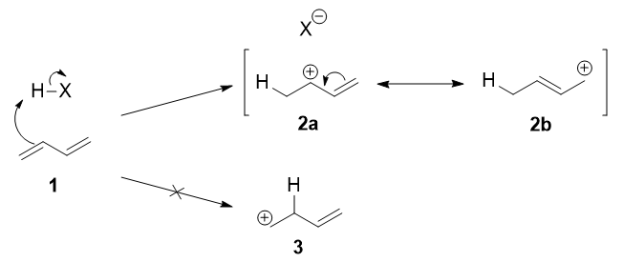
The more stable cation is not only secondary, but also allylic, and therefore enjoys stabilization via resonance. This is depicted in the resonance forms in 2a and 2b above. This allylic carbocation, more properly denoted as the resonance hybrid 2, has two carbons which have significant positive charge, and the bromide ion can attack either carbon. Attacking the central carbon, adjacent to the site of protonation, leads to the kinetic product 3 that is called as the 1,2-adduct; attacking the terminal carbon, distant from the site of protonation, leads to the thermodynamic product 4 that is called as the 1,4-adduct.