Unit-3
Physical, chemical and bacteriological examination of water and waste water
3.1.1 Temperature
Temperature is very important parameter because of its effect on chemical reactions on reaction rates, aquatic life, and the solubility of essential gases such as oxygen in water. The temperature of domestic wastewater is higher than that of the water supply, because of the addition of warm water from households. Depending on the geographical location, the mean annual temperature of wastewater varies from about 10 to 21.1 º C. The temperature of a wastewater sample can be measured with the help of ordinary mercury or digital thermometer.
Temperature is a measure of the average energy (kinetic) of water molecules. It is measured on a linear scale of degrees Celsius or degrees Fahrenheit.
It is one of the most important water quality parameters. Temperature affects water chemistry and the functions of aquatic organisms. It influences the:
3.1.2 pH
pH is a measure of how acidic or basic (alkaline) the water is (the term pH comes from the French: "puissance d'Hydrogène" which means strength of the hydrogen). It is defined as the negative log of the hydrogen ion concentration.
The pH scale is logarithmic and goes from 0 to 14. For each whole number increase (i.e. 1 to 2) the hydrogen ion concentration decreases tenfold and the water becomes less acidic. As the pH decreases, water becomes more acidic.
As water becomes more basic, the pH increases
3.1.3 Colour
By colour the quality of water can be judged. Pure water is colourless. In wastewater treatment, colour is not necessarily a problem, but instead is a indicator of the condition of the wastewater. Condition refers to the age of the wastewater, which is determined qualitatively by its colour and odour. Fresh wastewater is a light brownish-grey colour. The colour of wastewater changes sequentially from grey to dark grey and ultimately to black as the travel time in collection system increases (flow becomes increasingly more septic) and more anaerobic conditions develop.
3.1.4 Odour
In wastewater, odours are of major concern, especially to those who reside in close proximity to a wastewater treatment plant. These odours are generated by gases produced by decomposition of organic matter or by substances added to the wastewater. Odour from fresh wastewater is less objectionable than the odour from wastewater that has undergone anaerobic decomposition. The most characteristic odour of stale or septic wastewater is that of hydrogen sulphide (H2S), which is produced by anaerobic microorganisms that reduce sulphate to sulphide.
The malodorous compounds responsible for producing objectionable odours in water can be detected by diluting a sample with odour free water until the least detectable odour level is achieved. This is recorded as TON (Threshold Odour Number). The concentration of malodorous gases such as hydrogen sulphide, ammonia, mercaptans etc. emitted into the air from wastewater can be measured by any commercially available gas monitor.
3.1.5 Solids
Other than gases, all contaminants of water contribute to the solids content. Solids typically include inorganic matter such as silt, sand, gravel, and clay, and organic matter such as plant fibers and microorganisms from natural and manmade sources. Classified by their size and state, chemical characteristics, and size distribution, solids can be dispersed in water in both suspended and dissolved forms. In regards to size, solids in wastewater can be classified as suspended, settleable, colloidal, or dissolved. They are also characterized as being volatile or non-volatile.
There is different analytical procedure for analyzing solids in wastewater such as settling, filtration, and evaporation; because of their different particle sizes.
1. Total solids: - Total solids (TS) in wastewater are the amount of all solids, which are determined by drying a known volume of the sample in a pre weighed crucible dish at 105 °C. After cooling in an exsiccator, the crucible dish is again weighed. TS are determined by using the following formula:
TS = (M1 – M2)/V
Where,
M1: mass of crucible dish after drying at 105 °C (mg)
M2: mass of initial crucible dish (mg)
V: Volume of sample (L)
2. Volatile solids: -Volatile solids (VS) are the amount of solid that volatilizes when heated at 550 °C. This is a useful estimation for organic matter present in wastewater and is determined by burning the total solid at 550°C for about 2 hours in a muffle furnace. After cooling in an exsiccator to room temperature, it is weighed. VS are determined by using the following formula:
VS = (M1 – M3)/V
Where,
M1: mass of crucible dish after drying at 105 °C (mg)
M3: Mass of crucible dish after ignition at 550 °C (mg)
V: Volume of sample (L)
3. Fixed solids: - Fixed solids (FS) are the amount of solid that does not volatilize at 550 °C. This measure is used to gauge the amount of mineral matter in wastewater. It is the difference between TS and VS. It can be divided in a suspended and a filterable fraction.
4. Suspended solids: - Suspended solids (SS) are the solids retaining in a filter and is usually determined by filtration using glass fiber filters. In all analytical procedures for determination of suspended solids, weighed filters are used for sample filtration; the filters are dried at about 105°C after filtration, cooled in an exsiccate or to room temperature and the weight of the loaded filter is determined. SS is determined by using the following formula:
SS = (M4 – M5)/V
Where,
M4: mass of filter after drying at 105 °C (mg)
M5: mass of initial filter (mg)
V: Volume of sample (L)
5. Volatile suspended solids: - Volatile suspended solids (VSS) are, as indicated in figure 3, one portion of SS which are defined as that part of SS which can be removed by heating the solids at 550°C in a muffle furnace. The suspended solids is burned at 550°C for 2 hours in a muffle furnace and weighed after cooling in an exsiccator to room temperature. VSS is determined by using the following formula:
VSS = (M4 – M6)/V
M4: mass of filter after drying at 105 °C (mg)
M6: mass of filter after ignition at 550 °C (mg)
V: Volume of sample (L)
6. Fixed suspended solids: - Fixed suspended solids (FSS) are the solid that are unburnable at 550 °C and is determined by subtracting VSS from SS.
7. Dissolve solids: - Dissolve solids (DS) or filterable solids can be determined by subtracting SS from TS. The solids passing through the filter consist of colloidal and dissolved solids.
8. Settable solids: - Settable solids are those solids that will settle to the bottom of an Imhoff cone (a cone shaped container) in one hour and determined by allowing a wastewater sample to stand for one hour in an Imhoff cone which enables to read the volume of the settled solids. It is expressed as mL/L and is important, because it is related to the efficiency of sedimentation tank
3.1.6 Nitrogen
Nitrogen compounds with environmental relevance frequently analyted in wastewater are ammonia, nitrite, nitrate, and Kjeldahl nitrogen. Ammonia discharged to surface water can be nitrified in the aqueous environment if nitrifying microorganisms are present. The nitrifying bacteria consume dissolved oxygen for this process, thus depleting the oxygen content of the surface water with the consequence of massive dying of fish. Moreover, if the pH of the surface water is in the alkaline range, NH3 is formed which is toxic towards fish. The nitrate ion represents a nutrient leading to eutrophication of surface water, and nitrite is toxic and can react with amines (formed e.g. from amino acids of proteins) to yield N-nitrosamines which represent powerful carcinogens. Kjeldahl nitrogen is a sum parameter of compounds containing the nitrogen atom with an oxidation number of -3 (ammonia, amines and many other organic nitrogen compounds). It thus comprises organic nitrogen compounds besides ammonia nitrogen. This is also an important nitrogen parameter; because organic nitrogen compounds can be metabolized to ammonia (this conversion can also take place in surface water).
As many wastewater analyses (not only for nitrogen compounds) are photometric procedures, short information about photometry will be given. Photometry uses light as an analytical tool. As particular substances (analyses) absorb photons of different wavelengths to different extents, the wavelength (or colour) of the light applied for photometric analysis affects the specificity of the analytical procedure for a given analyte. The specificity can be increased by converting the analyte by reaction with certain reagents to form coloured products, because (besides the colour) also the reaction with a given reagent is specific for the analyte (other wastewater constituents would not react at all with the reagents used for conversion of a particular analyte). For example, ammonia can be converted to an intensely blue indophenols derivative by the following reactions:
NH4 + + OH-→ NH3 + H2O
NH3 + OCl-→ NH2Cl + OH-
NH2Cl + Phenol → Indophenol (intensely blue)
The last reaction is catalyzed by Mn2+ ions. For obtaining the blue product, an aliquot of the wastewater sample is mixed with a small volume of aqueous MnSO4 solution. Then the mixture is stirred and hypochlorous acid reagent and finally an alkaline aqueous phenol solution ("phenate reagent") are added. After 10 min the colour formation is complete for these particular reactions. The coloured product exhibits a maximum absorption at 630 nm (the complementary light causes the blue colour). The solution is transferred to a cuvette which is irradiated with light exhibiting a wavelength of 630 nm (satisfactory results are obtained in the 600 to 660 nm regions for this analytical procedure) and an intensity of Io in a photometer. In the photometer, the intensity of the light entering (Io) as well as the light leaving the cuvette (I) is determined (by means of a photodiode or a photomultiplier) as shown schematically in figure 6. The absorbance, i.e. log (Io/I), is linearly related to the Indophenol concentration as given by the Beer Lambert law:
Absorbance = log(Io/I) = 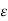 cd
cd
With the proportionality constant 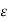 (molar absorptive or molar extinction coefficient), the length d of the way of the light through the cuvette (frequently 1 cm) and the molar concentration c of the coloured substance, resp. the concentration of the analyte in the sample (as one molecule of ammonia will yield one molecule of the coloured substance, the absorbance will also be linearly related to the ammonia concentration in the wastewater or in calibration solutions, resp.).
(molar absorptive or molar extinction coefficient), the length d of the way of the light through the cuvette (frequently 1 cm) and the molar concentration c of the coloured substance, resp. the concentration of the analyte in the sample (as one molecule of ammonia will yield one molecule of the coloured substance, the absorbance will also be linearly related to the ammonia concentration in the wastewater or in calibration solutions, resp.).
The colourless nitrite ion NO2- is also transformed to a coloured substance prior to photometric analysis. A standard method used for nitrite analysis suitable for determinations down to 1 µg NO2-N/l is the reaction of nitrite at pH 2 (formation of nitrous acid) with sulfanilic acid to give a diazonium salt which reacts with another reagent, (1-naphthyl)-ethylenediamine, in order to form a reddish purple azo dye that can be detected photometrically at 543 nm.
As for other analytes, also for nitrate determination several analytical methods can be applied. Greenberg et al. (1985) describe the chromotropic acid method as one of the possible procedures. Two molecules of nitrate react with one molecule of chromotropic acid (4,5-dihydroxy-2,7-naphthalene sulfonic acid) and the absorbance of the product is measured at 410 nm. The method interferes with nitrite. The nitrite ion is destroyed by reaction with urea which is also added to the test assay.
3.1.7 Phosphorus
Phosphorus is essential to the growth of algae and other biological organisms. The amount of phosphorus compounds present in wastewater discharge has to be controlled in order to avoid noxious algal blooms occurred in surface water. The usual forms of phosphorus found in aqueous solutions include the orthophosphate, polyphosphate, and organic phosphate. Three groups of phosphorus compounds have to be distinguished in phosphorus analysis of aqueous samples. The ortho-phosphate anion PO43-, poly- and metaphosphates which can be hydrolyzed to form ortho-phosphate, and phosphorus compounds which will not yield ortho-phosphate by hydrolysis but by oxidative treatment. The latter group is mainly represented by organic phosphorus compounds. The sum of all three phosphorus species is designated as total phosphorus.
The ortho-phosphate anion is again determined by photometry after it has been transformed by addition of ammonium molybdate, potassium antimonyl tartrate and ascorbic acid to yield the intensely blue compound "molybdenum blue" which is quantified by means of a photometer at 880 nm using phosphate calibration solutions of known phosphate concentrations.
The sum of ortho-phosphate and acid-hydrolysable phosphorus is determined nearly in the same way except a hydrolysis step prior to quantification of original and hydrolysis generated ortho-phosphate. The hydrolysis is performed by gentle boiling of the wastewater sample after addition of a mixture of concentrated H2SO4 and concentrated HNO3. After cooling and neutralization with NaOH solution, the ortho-phosphate can be analyzed following the procedure given above.
Determination of total phosphorus requires oxidation as well as hydrolysis prior to orthophosphate analysis. This is realized by boiling the wastewater sample after addition of concentrated HNO3, evaporation on a steam bath, addition of 70% perchloric acid and concentrated HNO3, boiling until the mixture clears.
3.1.8 Chlorides
Domestic wastewater is a rich source of chlorides, because human excreta, mainly urine, are rich in chloride. It does not present a major pollution threat. But, Chloride ion concentration is an important factor to be considered if treated effluent is used for irrigation. High chloride concentration disturbs the osmotic balance between the plants and the soil, which affects the growth of the plants. The level of chlorides in wastewater sample is determined by the titration of the sample with mercuric nitrate in the presence of an indicator.
3.1.9 Toxic Metals & Compounds
For the determination of metals, there exist special methods as flame emission photometry (e.g. important for the fertilizer component potassium). In this procedure the aqueous sample is transferred into a flame where the metals are electronically excited resulting in an emission of light of a particular wavelength. This emission can be detected and used for quantification of the concerning metal ion.
A similar method is also useful for the determination of some toxic heavy metals (atomic emission spectrometry/inductively coupled plasma, AES/ICP). The aqueous solution is pumped into a small plasma generated by high frequency fields where the metals are electronically excited leading to emission of light of that wavelength which is characteristic for the particular metal of concern. With this method, several metals can be determined simultaneously.
On the other hand, aqueous solutions of metal salts can also absorb distinct wavelengths of light, when they are heated to very high temperatures (flame or graphite furnace) and converted from ions to atoms by this. The light absorbed by the atoms can be used for quantification of particular metal ions in aqueous solutions like wastewaters. The method is called atomic absorption spectrometry (AAS). Solids have to be digested prior to AAS analysis if their metal content is to be analyzed. Details for such methods can be read in the "Standard Methods".
Sometimes, there is interest in the concentrations of particular organic compounds contained in wastewaters. For such analyses, gas chromatography is a useful tool, but very complex in execution. For many gas chromatographic methods, wastewater samples have to limits for particular trace organics. And the final concentrate is then analyzed. A very small volume of the concentrate (in the range of one µl) is transferred to the so-called injector of the gas chromatograph by a syringe. The injector is heated to temperatures in the range of 200°C and flushed by t he inert carrier gas (very often helium is used). At these high temperatures the total solution evaporates at once and the analytes as well as the extractant are transported by the carrier gas to a separation device, the so-called column. The column is usually a capillary made of fused silica (a material that has substituted glass which had been used earlier for manufacturing capillaries for gas chromatography) of some 10 m length. The inner wall of the capillary is lined by thin films of particular polymers which control the separation characteristics of the column. Different analytes (as well as the extractant) show different interactions with the polymer film material and thus exhibit different velocities passing the column. The temperature of the column also affects separation of analytes and varies - depending on the separation problem - between room temperature and around 300°C. It can also be changed during the chromatographic run ("temperature program"). At the end of the column the carrier gas (and the analytes as well as the extractants arriving at different times) are detected by devices like flame ionization or electron capture detectors giving signals which are related to the concentrations of the analytes in the extract. Very useful are mass spectrometers for detection, because the detected mass fragments of the analytes can serve as "fingerprints" resulting in identification of particular organic compounds after comparison to computerized mass spectra of known organics.
3.1.10 Biochemical Oxygen Demand (BOD)
Biochemical Oxygen Demand is a sum parameter and the amount of oxygen required to oxidize organic matter present in the water biochemically. So BOD is an indirect measure of the concentration of organic contamination in water. BOD analysis does not oxidize all of the organic matter present in the waste; only the organics that are biochemically degradable during n days time period at 20°C are oxidized. The day period is given as index in BODn. The standard for usual measurements is a 5-day period.
BOD5 is the most widely used parameter of organic pollution applied to wastewater and is used:
For the measurement of BOD, different volumes of wastewater are mixed in special BOD bottles with a liquid called "dilution water". This may be final effluent of a wastewater treatment plant which still contains some microorganisms or primary clarifier effluent diluted with tap water; it has to be supplemented with nitrogen, e.g. urea, and phosphate, aerated for a period of 3 to 10 days prior to use for BOD analysis, which had been saturated with oxygen prior to BOD analysis by bubbling in air.
The BOD bottles are completely filled and sealed with a glass stopper in such a way that no more air bubbles are contained in the bottles. With every mixture a duplicate of bottles is prepared. In one bottle of each pair, the concentration of dissolved oxygen is determined (e.g. by means of an oxygen probe) immediately after mixing. The other bottle is stored for n days at 20°C in the dark (to prevent photochemical reactions). At the end of this period, the concentration of dissolved oxygen is measured also in this bottle. The difference of oxygen concentration in the two bottles of a pair is the oxygen consumption (OC) (mg O2/l). From the oxygen consumption of a particularly diluted wastewater sample and the oxygen consumption of the blanks (OCDW), the BODn is calculated as follows:
BODn = DF X OC – (DF-1) X OCDW
Where,
DF =dilution factor = (V(diluted sample)/V (sample before dilution).
BOD values determined for different dilutions should give a straight line when drawn as a function of the term V(sample before dilution). When points corresponding to low dilution factors (i.e. to high V(sample before dilution)) in this graph are lying below the extrapolated line, this is a hint that inhibition of microorganisms occurred in samples with low dilution. These values must not be applied for BOD determination.
3.1.11 Chemical Oxygen Demand (COD)
The equivalent amount of oxygen required to oxidize organic matter present in a water sample by means of a strong chemical oxidizing agent is called chemical oxygen demand (COD). COD is also a sum parameter and is used to measure the content of organic matter of wastewater. The COD values include the oxygen demand created by biodegradable as well as non-biodegradable substances. As a result, COD values are greater than BOD. In comparison with BOD5, COD measurement has an advantage in that it requires a short digestion period of about 3 hours rather than incubation of 5 days period required for BOD5 measurement.
In the COD method, not the product (CO2) formed by oxidation of the organic wastewater constituents is measured, but the consumption of the oxidant (calculated as oxygen O2). Thus, an exact amount of oxidant has to be used for the oxidation of the organics in a given volume of a wastewater sample, and the excessive oxidant which is not consumed for complete oxidation of organics must be quantified. Complete oxidation postulates that the only oxidation product formed is CO2 and not any organic intermediate with high carbon oxidation numbers. Although COD is given as mg of consumed oxygen per litre of wastewater, the oxidant used in the analytical procedure is not oxygen, but potassium dichromate (K2Cr2O7) in concentrated sulfuric acid. The substance K2Cr2O7 is a powerful oxidant in an acid milieu (H2Cr2O7 is formed) at elevated temperature (148°C). Oxidation is the abstraction of electrons from a substance that is oxidized. As one molecule K2Cr2O7 can accept 1.5 times more electrons than the molecule O2, this is considerate by calculation.
The analytical standard procedure prescribes to place 50 ml of the wastewater sample in a 500-ml-refluxing flask, to add 1 g of HgSO4, 5 ml of a mixture of Ag2SO4 (which serves as a catalyst for the oxidation of the organics) in concentrated sulfuric acid, to add subsequently 25 ml of a solution of the oxidant K2Cr2O7) in concentrated sulfuric acid and to heat the mixture under reflux after vigorous mixing for two hours. The K2Cr2O7 that has not been consumed for oxidation is then quantified by titration with an aqueous solution of Fe(NH4)2SO4 with known concentration. The residual K2Cr2O7 oxidizes the Fe2+ of the titrant Fe(NH4)2SO4 to give Fe3+. When all the residual K2Cr2O7 is consumed (reduced by Fe22+), the indicator ferroin, which has to be added to the wastewater/K2Cr2O7 mixture prior to titration, turns from blue-green to reddish brown. At this end-point of titration, the volume of the Fe(NH4)2SO4is read from the burette and the residual amount of the oxidant K2Cr2O7 after oxidizing the organic constituents in the wastewater sample can be calculated. By this, the consumption of K2Cr2O7 during oxidation and its oxygen equivalent are calculated, giving the COD.
Besides problems with working safety (use of the carcinogenic K2Cr2O7), there are also some analytical problems, because K2Cr2O7 does not only oxidize organic, but also some inorganic molecules or ions. Chloride, which is a normal constituent of wastewaters, is oxidized by K2Cr2O7 forming Cl2 gas. In order to prevent oxidation of chloride, it is masked by the addition of HgSO4. Chloride bound to Hg2+ is not oxidized by K2Cr2O7. However, the addition of mercury sulfate to all samples for COD determinations generates a large amount of toxic waste in laboratories for wastewater analyses (some laboratories purify this liquid waste stream by ion exchange giving the ion exchange regenerates to recycling companies for mercury and also for silver and chromium recovery). If the chloride concentration in the wastewater sample exceeds 1 g/l, the chloride has to be removed prior to COD analysis from the sample by heating it after addition of sulfuric acid and removing the formed hydrochloric acid from the gas phase by absorption to alkaline materials. But there are other substances which can cause troubles with the COD analysis: If the wastewater contains e.g. bromide, iodide, sulfite, Fe2+, Co+ or hydrogen peroxide, these reducing agents will also be oxidized by K2Cr2O7. This K2Cr2O7 consumption, however, is not caused by organics leading to misinterpretations about the content of organics of the wastewater. Another problem is that not every organic substance is completely oxidized under the conditions of COD analysis. Many nitrogen-containing heterocycles (e.g. pyridine) consume significantly less K2Cr2O7 than theoretically assumed.
Key Takeaways:
For any water body to function adequately in satisfying the desired use, it must have corresponding degree of purity. Drinking water should be of highest purity. As the magnitude of demand for water is fast approaching the available supply, the concept of management of the quality of water is becoming as important as its quantity.
Each water use has specific quality need. Therefore, to set the standard for the desire quality of a water body, it is essential to identify the uses of water in that water body. In India, the Central Pollution Control Board (CPCB) has developed a concept of designated best use. According to this, out of the several uses of water of a particular body, the use which demands highest quality is termed its designated best use. Five designated best uses have been identified. This classification helps the water quality managers and planners to set water quality targets and design suitable restoration programs for various water bodies.
3.2.1 Designated Best Uses of Water
Designated Best Use | Class | Criteria |
Drinking Water Source without conventional treatment but after disinfection | A | 1.Total Coliforms Organism MPN/100ml shall be 50 or less 2. pH between 6.5 and 8.5 3. Dissolved Oxygen 6mg/l or more 4. Biochemical Oxygen Demand 5 days 20 C, 2mg/l or less |
Outdoor bathing (Organized) | B | 1.Total Coliforms Organism MPN/100ml shall be 500 or less 2. pH between 6.5 and 8.5 3. Dissolved Oxygen 5mg/l or more 4. Biochemical Oxygen Demand 5 days 20 C, 3mg/l or less |
Drinking water source after conventional treatment and disinfection | C | 1. Total Coliforms Organism MPN/100ml shall be 5000 or less 2. pH between 6 and 9 3. Dissolved Oxygen 4mg/l or more 4. Biochemical Oxygen Demand 5 days 20 C, 3mg/l or less |
Propagation of Wild life and Fisheries | D | 1. pH between 6.5 and 8.5 2. Dissolved Oxygen 4mg/l or more 3. Free Ammonia (as N) 4. Biochemical Oxygen Demand 5 days 20 C, 2mg/l or less |
Irrigation, Industrial Cooling, Controlled Waste disposal | E | 1. pH between 6.0 and 8.5 2. Electrical Conductivity at 25 C micro mhos/cm, maximum 2250 3. Sodium absorption Ratio Max. 26 4. Boron Max. 2mg/l |
| Below-E | Not meeting any of the A, B, C, D & E criteria |
A colour coding frequently used to depict the quality of water on maps
Blue water | This water can be directly used for drinking, industrial use, etc. |
Green water | Water contained in soil and plants is termed as green water |
White water | Atmospheric moisture is white water |
Brown or grey water | Various grades of wastewater are shown by brown or grey colour |
In India, CPCB has identified water quality requirements in terms of a few chemical characteristics, known as primary water quality criteria. Further, Bureau of Indian Standards has also recommended water quality parameters for different uses in the standard ARE 2296:1992.
3.2.2 Water Quality Standards in India (Source IS 2296:1992)
Characteristics | Designated best use | ||||
A | B | C | D | E | |
Dissolved Oxygen (DO)mg/l, min | 6 | 5 | 4 | 4 | - |
Biochemical Oxygen demand (BOD)mg/l, max | 2 | 3 | 3 | - | - |
Total coliform organisms MPN/100ml, max | 50 | 500 | 5,000 | - | - |
pH value | 6.5-8.5 | 6.5-8.5 | 6.0-9.0 | 6.5-8.5 | 6.0-8.5 |
Colour, Hazen units, max. | 10 | 300 | 300 | - | - |
Odour | Un-objectionable |
| - | - | |
Taste | Tasteless | - | - | - | - |
Total dissolved solids, mg/l, and max. | 500 | - | 1,500 | - | 2,100 |
Total hardness (as CaCO3), mg/l, and max. | 200 | - | - | - | - |
Calcium hardness (as CaCO3), mg/l, max. | 200 | - | - | - | - |
Magnesium hardness (as CaCO3), mg/l, max. | 200 | - | - | - | - |
Copper (as Cu), mg/l, max. | 1.5 | - | 1.5 | - | - |
Iron (as Fe), mg/l, max. | 0.3 | - | 0.5 | - | - |
Manganese (as Mn), mg/l, max. | 0.5 | - | - | - | - |
Chlorides (as Cu), mg/l, max. | 250 | - | 600 | - | 600 |
Sulphates (as SO4), mg/l, max. | 400 | - | 400 | - | 1,000 |
Nitrates (as NO3), mg/l, max. | 20 | - | 50 | - | - |
Fluorides (as F), mg/l, max. | 1.5 | 1.5 | 1.5 | - | - |
Phenolic compounds (as C2H5OH), mg/l, max. | 0.002 | 0.005 | 0.005 | - | - |
Mercury (as Hg), mg/l, max. | 0.001 | - | - | - | - |
Cadmium (as Cd), mg/l, max. | 0.01 | - | 0.01 | - | - |
Selenium (as Se), mg/l, max. | 0.01 | - | 0.05 | - | - |
Arsenic (as As), mg/l, max. | 0.05 | 0.2 | 0.2 | - | - |
Cyanide (as Pb), mg/l, max. | 0.05 | 0.05 | 0.05 | - | - |
Lead (as Pb), mg/l, max. | 0.1 | - | 0.1 | - | - |
Zinc (as Zn), mg/l, max. | 15 | - | 15 | - | - |
Chromium (as Cr6+), mg/l, max. | 0.05 | - | 0.05 | - | - |
Anionic detergents (as MBAS), mg/l, max. | 0.2 | 1 | 1 | - | - |
Barium (as Ba), mg/l, max. | 1 | - | - | - | - |
Free Ammonia (as N), mg/l, max | - | - | - | 1.2 | - |
Electrical conductivity, micromhos/cm, max | - | - | - | - | 2,250 |
Sodium absorption ratio, max | - | - | - | - | 26 |
Boron, mg/l, max | - | - | - | - | 2 |
3.2.3 Drinking Water Specifications (IS 10,500:1991)
Characteristics | Desirable limit | Permissible limit |
Essential Characteristics | ||
Colour, Hazen Units, Max | 5 | 25 |
Odour | Unobjectionable | - |
Taste | Agreeable | - |
Turbidity, NTU, Max | 5 | 10 |
PH value | 6.5 to 8.5 | - |
Total Hardness (as CaCo3), mg/l, Max | 300 | 600 |
Iron (as Fe), mg/l, Max | 0.3 | 1.0 |
Chlorides (as Cl), mg/l, Max | 250 | 1,000 |
Residual free chlorine, mg/l, Max | 0.2 | - |
Desirable Characteristics | ||
Dissolved solids, mg/l, Max | 500 | 2,000 |
Calcium as (Ca), mg/l, Max | 75 | 200 |
Magnesium (as Mg), mg/l, Max | 30 | 75 |
Copper (as Cu), mg/l, Max | 0.05 | 1.5 |
Manganese (as Mn), mg/l, Max | 0.1 | 0.3 |
Sulphate (as So4), mg/l, Max | 200 | 400 |
Nitrate (as No3), mg/l, Max | 45 | 100 |
Fluoride (as F0, mg/l, Max | 1.0 | 1.5 |
Phenolic compounds (as C6H5OH), mg/l, Max | 0.001 | 0.002 |
Mercury (as Hg), mg/l, Max | 0.001 | - |
Cadmium (as Cd), mg/l, Max | 0.01 | - |
Selenium (as Se), mg/l, Max | 0.01 | - |
Arsenic (as As), mg/l, Max | 0.05 | - |
Cyanide (as CN), mg/l, Max | 0.05 |
|
Lead (as Pb), mg/l, Max | 0.05 | - |
Anionic detergents (as MBAS), mg/l, Max | 0.02 | 1.0 |
Chromium (as Cr6+), mg/l, Max | 0.05 | - |
PAH, mg/l, Max | - | - |
Mineral oil, mg/l, Max | 0.01 | 0.03 |
Pesticides, mg/l, MAX | Absent | 0.001 |
Alkalinity, mg/l, Max | 200 | 600 |
Aluminum (as Al), mg/l, Max | 0.03 | 0.2 |
Boron, mg/l, Max | 1 | 5 |
Key Takeaways:
3.3.1 Drinking water quality standard
A. Bacteriological parameter
Parameters | Units | Concentration |
Faecal Coliform | MPN/100ml | 0 |
Total Coliform | MPN/100ml | < 2.2 |
Enterovirus | MPN/100ml | 0 |
B. Physical-Chemical parameters
No | Parameters | Unit | Concentration | |
Minimum | Maximum | |||
1 | Aluminum | mg/l | 0.1 | 0.2 |
2 | Ammonia | mg/l | 0.5 | 1.5 |
3 | Chloride | mg/l | 200 | 250 |
4 | Copper | mg/l | 1.0 | 2.0 |
5 | Iron | mg/l | 0.3 | <1 |
6 | Manganese | mg/l | 0.1 | 0.5 |
7 | Sodium | mg/l | 200 | 250 |
8 | Sulphate | mg/l | 200 | 250 |
9 | Hydrogen Sulphide | mg/l | 0.05 | 0.1 |
10 | Conductivity | us/cm | - | <1,000 |
11 | Total dissolved solids | mg/l | 500 | 600 |
12 | Sodium Chloride | mg/l | 100 | 300-350 |
13 | Potential of Hydrogen | - | 6.5 | 8.5 |
14 | Temperature | °C | 25 | 35 |
15 | Hardness | mg/l | 50 | 300 |
16 | Turbidity | NTU | - | <10 |
17 | Taste and Odour | - | - | Acceptable |
18 | Colour | TCU | - | 5 |
19 | Residual Chloride (if Chlorine disinfection is used) | mg/l | - | <0.2 |
C. Health significant chemical parameters
No | Parameters | Unit | Maximum Concentration |
1 | Antimony | mg/l | 0.005 |
2 | Arsenic | mg/l | 0.01 - 0.05 |
3 | Barium | mg/l | 0.7 |
4 | Boron | mg/l | 0.5 |
5 | Cadmium | mg/l | 0.003 |
6 | Chromium | mg/l | 0.05 |
7 | Cyanide | mg/l | 0.07 |
8 | Fluoride | mg/l | 1.5 |
9 | Lead | mg/l | 0.01 |
10 | Mercury | mg/l | 0.001 |
11 | Nitrate | mg/l | 50 |
12 | Nitrite | mg/l | 3 |
13 | Selenium | mg/l | 0.01 |
D. Priority parameters
No | Parameters | Unit | Maximum Concentration |
1 | Iron | mg/l | <1 |
2 | Manganese | mg/l | <0.5 |
3 | Arsenic | mg/l | <0.05 |
4 | Fluoride | mg/l | <1.5 |
5 | Nitrate | mg/l | 50 |
6 | Nitrite | mg/l | 3 |
7 | Nitrite Nitrogen | mg/l | 1 |
8 | Potential of Hydrogen | - | 6.5 - 8.5 |
9 | Coliform | MPN/100ml | 0 |
10 | Conductivity | us/cm | 1000 |
11 | Residual Chloride | mg/l | 0.2 |
12 | Total Hardness | mg/l | <300 |
13 | Turbidity | NTU | <10 |
14 | Taste and Odour | - | Acceptable |
3.3.2 Ground water quality standard
No | Substances | Unit | Standard | Method of Measurement |
I. Volatile Organic Compound | ||||
1 | Benzene | mg/l | 0.005 | Purge and Trap Gas Chromatography or Purge and Trap Gas Chromatography/Mass Spectrometry |
2 | Carbon Tetrachloride | mg/l | 0.005 | |
3 | 1.2-Dichloroethane | mg/l | 0.005 | |
4 | 1.1-Dichloroethylene | mg/l | 0.007 | |
5 | Cis-1.2-Dichloroethyline | mg/l | 0.070 | |
6 | Trans-1.2-Dichloroethylene | mg/l | 0.1 | |
7 | Dichloromethane | mg/l | 0.005 | |
8 | Ethyl benzene | mg/l | 0.7 | |
9 | Styrene | mg/l | 0.1 | |
10 | Tetrachloroethylene | mg/l | 0.005 | |
11 | Toluene | mg/l | 1 | |
12 | Trichloroethylene | mg/l | 0.005 | |
13 | Toluene | mg/l | 0.2 | |
14 | Trichloroethylene | mg/l | 0.005 | |
15 | Total Xylenes | mg/l | 10 | |
II. Heavy Metals | ||||
1 | Cadmium | mg/l | 0.003 | Direct Aspiration/ Absorption Spectrometry or Inductively Coupled Plasma/Plasma Emission Spectroscopy |
2 | Hexavalent Chromium | mg/l | 0.05 | |
3 | Copper | mg/l | 1 | |
4 | Lead | mg/l | 0.01 | |
5 | Manganese | mg/l | 0.5 | |
6 | Nickel | mg/l | 0.02 | |
7 | Zinc | mg/l | 5 | |
8 | Arsenic | mg/l | 0.01 | Hydride Generation/ Atomic Absorption Spectrometry of Inductively Coupled Plasma / Plasma Emission Spectroscopy |
9 | Selenium | mg/l | 0.01 | |
10 | Mercury | mg/l | 0.001 | Cold-Vapour Atomic Absorption Spectrometry / Plasma Emission Spectroscopy |
III. Pesticides | ||||
1 | Chlordane | mg/l | 0.0002 | Liquid-Liquid Extraction |
2 | Dieldrin | mg/l | 0.00003 | Gas Chromatography / Mass Spectrometry or Liquid-Liquid Extraction Gas Chromatography (Method I) |
3 | Heptachlor | mg/l | 0.0004 | |
4 | Heptachlor Epoxide | mg/l | 0.0002 | |
5 | DDT | mg/l | 0.002 | |
6 | 2,4D | mg/l | 0.03 | Liquid-Liquid Extraction |
7 | Atrazine | mg/l | 0.003 | |
8 | Lindane | mg/l | 0.0002 | Liquid-Liquid Extraction |
9 | Pentachlorophenol | mg/l | 0.001 | Liquid-Liquid Extraction |
IV. Others | ||||
1 | Benzo (a)pyrene | mg/l | 0.0002 | Liquid-Liquid Extraction |
2 | Cyanide | mg/l | 0.2 | Pyridine Barbituric Acid or Colorimetric or Iron Chromatography |
3 | PCBs | mg/l | 0.0005 | Liquid-Liquid Extraction |
4 | Vinyl Chloride | mg/l | 0.002 | Purge and Trap Gas Chromatography or Purge and Trap Gas Chromatography/Mass Spectrometry |
3.3.3 General industrial waste water discharge standard
No | Parameters | Unit | Maximum Concentration |
1 | BOD5 | mg/l | 40 |
2 | Ammonia Nitrogen | mg/l | 4 |
3 | Total Suspended Substances | mg/l | 40 |
4 | Potential of Hydrogen | - | 6-9.5 |
5 | Total Dissolved Substances | mg/l | 3,500 |
6 | Phenols | mg/l | 0.3 |
7 | Phosphorous | mg/l | 1.0 |
8 | Silver | mg/l | 0.1 |
9 | Zinc | mg/l | 1.0 |
10 | Sulphide | mg/l | 1.0 |
11 | Free Chlorine | mg/l | 1.0 |
12 | Chloride | mg/l | 500 |
13 | Iron | mg/l | 2.0 |
14 | Fluoride | mg/l | 15 |
15 | Cyanide | mg/l | 0.1 |
16 | Copper | mg/l | 0.5 |
17 | Lead | mg/l | 0.2 |
18 | Oil and Grease | mg/l | 5 |
19 | Nickel | mg/l | 0.2 |
20 | Mercury | mg/l | 0.005 |
21 | Manganese | mg/l | 1.0 |
22 | Arsenic | mg/l | 0.25 |
23 | Barium | mg/l | 1.0 |
24 | Cadmium | mg/l | 0.03 |
25 | Chromium | mg/l | 0.1 |
26 | Total Chromium | mg/l | 0.5 |
Key Takeaways:
The methods of sewage disposal can classify as follows:
3.4.1 Disposal by dilution
In this process, the raw sewage or the partially treated sewage is thrown into natural waters having large volume. The sewage in due course of time is purified by what is known as the self-purification capacity of natural waters. The limit of discharge and degree of treatment of sewage are determined by the capacity of self-purification of natural waters.
3.4.1.1 Conditions favorable for dilution
Following conditions are favorable for sewage to be disposed of by dilution into natural waters
3.4.1.2 Types of natural waters
Following are the natural waters into which the sewage can be discharged for dilution
3.4.1.3 Self-purification of natural waters
When sewage is discharged into natural water, its organic matter gets oxidized by the dissolved oxygen content in water. The oxidation of organic matter converts such matter into simple inoffensive substances. Deficiency of dissolved oxygen thus created in natural waters is filled up by the absorption of atmospheric oxygen. Thus, the oxygen of water is consumed by sewage and at the same time, it is replenished by the atmosphere. This phenomenon which occurs in all-natural waters is known as self-purification of natural waters. It is thus seen that natural waters, polluted by sewage, are purified in natural course by the phenomena of self-purification.
The rate of self-purification will depend on various factors such as rate of re-aeration type of organic matter present in sewage, temperature, velocity of flow, presence of available oxygen in receiving waters, sedimentation, etc.
3.4.2 Disposal by land treatment
Here, the raw domestic waste water (sewage) is applied on the land. A part of sewage evaporates and the remaining portion percolates through the ground and is caught by the underground drains for disposal into natural waters. The sewage adds to the fertilizing value of land and crops can be profitably raised on such land. The term sewage farming is also sometimes used for indicating disposal of sewage by land treatment. The design of a good land treatment system demands the services of environmental engineers, hydraulic engineers, irrigation engineers, agronomists, soil scientist, etc.
3.4.2.1 Conditions favorable for land treatment
3.4.2.2 Advantages of land treatment
3.4.2.3 Disadvantages of land treatment
3.4.3 Preventive measures
In order to prevent sewage sickness of land, the following preventive measures may be adopted
1. Alternative arrangement: There should be ample provision of extra land so that land with sewage sickness can be given the desired rest. Alternatively, sewage should be disposed of by some other method when sewage farms are taking rest
2. Depth of sewage: If sewage is applied in excess, the chances of sewage sickness are increased. The land is unable to receive the excess sewage in a satisfactory way and it ultimately clogs up. Depth of sewage on land should be carefully decided by keeping in view the climatic conditions, drainage facilities, nature of crops and characteristics of soil.
3. Drainage of soil: Subsoil drain pipes should be laid in sufficient number to collect the percolated effluent
4. Intermittent application: Sewage should be applied on land at intervals. The period between successive applications depends on general working of sewage farm and the permeability of soil. Depending on the nature of the soil, this period between successive applications varies from few hours to few weeks.
5. Pretreatment of sewage: sewage should be given some pretreatment before it is applied on land.
6. Rotation of crops: It is desirable to grow different types of crops on a piece of land instead of one single crop. Rotation of crops minimizes the chances of sewage sickness.
7. Treatment to land: The land affected by sewage sickness should be properly treated before it is put up in use again. Clogged surfaces should be broken by suitable equipment.
Key Takeaways:
References:
1. Manual on Water Supply and Treatment, C. P. H. E. E. O., Ministry of Urban Development,
Government of India, New Delhi
2. Manual on Sewerage and Sewage Treatment, C. P. H. E. E. O., Ministry of Urban
Development, Government of India, New Delhi
3. Steel and McGhee: Water Supply and Sewerage
4. Fair and Geyer: Water Supply and Wastewater Disposal
5. Hammer and Hammer Jr.: Water and Wastewater Technology
6. Raju: Water Supply and Wastewater Engineering
7. Rao: Textbook of Environmental Engineering
8. Davis and Cornwell: Introduction to Environmental Engineering
9. Kshirsagar: Water Supply and Treatment and Sewage Treatment Vol. I and II
10. Punmia: Water Supply and Wastewater Engineering Vol. I and II
11. Birdie: Water Supply and Sanitary Engineering
12. Ramalho: Introduction to Wastewater Treatment Processes
13. Davis Mackenzie L., Cornwell, David A., “Introduction to Environmental Engineering”
McGraw Hill Education (India) Pvt. Ltd., New Delhi.
14. Birdie: Water Supply and Sanitary Engineering
15. Ramalho: Introduction to Wastewater Treatment Processes
16. Parker: Wastewater Systems Engineering
17. A.K. Jain, Environmental Engineering, Khanna Publishing House