Unit-4
Objectives of water treatment
4.1.1 Unit operations
Unit operations are the physical operations to remove the impurities present in the water and waste water where as the unit processes are the chemical and biological conversion on the status of the impurities that they will be converted to a form that can be easily separated. Both are applied especially to make the fine colloidal particles to coalesce and grow in size to be removed from the water or waste water. There is no impurity that can be categorized as inorganic, it is named so for it takes time to disintegrate and had been to this hard form, free from decomposable matter with the ecological factors. We can find metal eating bacteria these days that makes the accelerated form to human use get decelerated to favour nature accommodate effectively as indigenous.
1. Screens: -Screens are in use from the intake structure where they prevent the floating matter to enter into the pumping units, and fine and coarse screens are in use to treat waste water to prevent the entry of floating wastes and coarse solids into the treatment.
2. Sedimentation: -Sedimentation is simply detaining water for a sufficient time mostly in stagnant or relatively stagnant position to make the flow velocity of water less than the settling velocity of the solid particles that they without being driven by horizontal force settles down by gravity. The efficiency of the process depends on the detention time, how long the waste water remains within the sedimentation tank.
3. Coagulants: -Coagulants are added to the water that the flocculent particles grow bigger in size which is by chemical reaction by rapid mixing and slow mixing and the coalescent particles which grew in size gets removed by settling.
4. Filtration: -Filtration is to the removal of fine particle sand dissolved solids where the fine sand layer and coarse sand layer below serves as the media to remove colloidal solids and the water remains completely free of solids.
The unit operations and processes can be applied in individual units of houses, colonies and industries that it gives fewer problems to the environment and handled with more efficiency.
Key Takeaways
4.1.2 Processes
The objective of municipal and industrial waste water treatment is to extract pollutants, remove toxicants, neutralise coarse particles, kill pathogens so that quality of discharged water is improved to reach the permissible level of water to be discharged into water bodies or for agricultural land.
Treatment of water thus aims at reduction of BOD, COD, eutrophication etc. of receiving water bodies and prevention of bio-magnification of toxic substances in food chain.
4.1.2.1 Steps Involved in Waste Water Treatment:
1. Preliminary Treatment
Waste Water Treatment: Objective and Steps
Steps Involved in Waste Water Treatment:
Various steps involved in treatment of waste water are as follows:
1. Preliminary TreatmentScreening:
In this treatment debris, gross solids, grit, oil and grease are removed by passing waste water through screens, grit chambers and skimming tanks.
2. Primary TreatmentPrimary treatment of sewage removes 60% suspended solids, 30% COD, 35% BOD, 10% P and 20% total nitrogen.
It includes the following processes:
(i) Sedimentation
About 50% suspended solids can be removed by gravitational settling under quiescent conditions.
(ii) Mechanical Flocculation and Coagulation
Fine suspended solids and colloidal particles are removed by passing waste water through clariflocculator and using coagulants like alum and poly-electrolytes.
(iii) Neutralization
Highly acidic and alkaline waste waters are neutralised by lime slurry or NaOH and H2SO4 or CO2 respectively.
3. Secondary (Biological) TreatmentThe dissolved and colloidal organic matter in waste water/sewage is removed by aerobic or anaerobic processes. The effluent from primary sedimentation tank is first subjected to aerobic oxidation in processes such as aerated lagoons, trickling filters, activated sludge units, oxidation ponds etc.
Then the sludge obtained in these aerobic processes, together with that obtained in the primary sedimentation tank, is subjected to anaerobic digestion in the sludge digester (Fig. 2). Secondary treatment removes about 80% COD, 90% BOD, 30% P, 50% total N and oil, grease, phenol, grit, scum etc.
4. Tertiary TreatmentTertiary treatment is the final treatment meant for abolishing the secondary effluents and removal of fine suspended solids, traces of organics and bacteria. The sewage effluent from secondary treatment plant is introduced into a flocculation tank where lime is added to eliminate calcium phosphate.
The solution then enters the NH3 stripping tower. Nitrogen present in waste water exists as NH+4 which are converted to gaseous ammonium ion at high pH (ll). Phosphorus is removed by adding ferric chloride or aluminium sulphate. The remaining organic materials are removed by desalination, ion exchange and finally chlorination is used for disinfection.
The toxic, non-biodegradable chemicals in industrial waste water can be removed by adsorption (on activated charcoal), ion exchange, ultra-filtration, reverse osmosis and electro dialysis.
Key Takeaways
4.1.3 Flow sheets
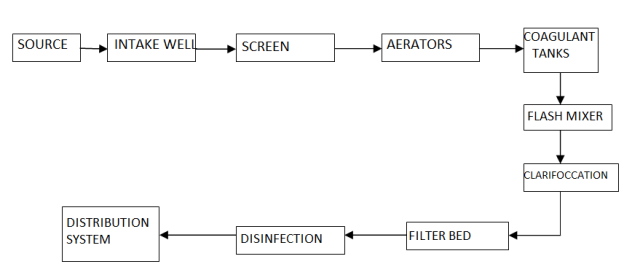
4.1.3.1 Functions of each unit
Key Takeaways
4.2.1 Screening
It is the first operation in wastewater treatment to remove most of the bigger and longer visible objects such as trees, branches, sticks, rags, boards, animals etc present in raw water of surface water sources as the screens protect pumps and other mechanical equipment’s and to prevent clogging of valves and other appurtenances.
4.2.1.1 Types of Screens
Screens are of three types depending upon the size of opening-
a) Coarse screens
b) Medium screens
c) Fine screens
a) Coarse Screens - They are also known as racks. Spacing between bars is 50 mm or more than this. These screens help in removing large floating objects from sewage. The material separated by coarse screens consists of rags, woods, sticks and paper etc. which will putrefy and must therefore be disposed of by incineration, burial or dumping.
Coarse screens consist of parallel iron rods placed vertically or at a slight slope of about 2 to 10 cm centre to centre. The coarse screens are also now normally kept inclined at about 45-60° so as to increase the opening area to reduce the flow velocity and thus making the screening more effective. Velocity of wastewater through screen should not be more than 0.8 to 1 m/sec. The material which is collected on the upstream side of screens is removed either manually or mechanically. In mechanically cleaned screens, a rack traverses the front of the screen either continuously or intermittently. Mechanical cleaning is done at large plants with mechanically operated rakes while manual cleaning is done at small plants with hand operated rakes.
b) Medium Screens - In this type of screen, spacing between the bars is 6-40 mm. These screens will ordinarily collect 30-90 litres of material per million litre of Sewage. Screenings (material removed by screens) usually contain some quantity of organic material which may putrefy and become offensive, therefore, be disposed of by incineration or burial (not by dumping).
Rectangular shaped coarse and medium screens, made up of steel are now-a-days widely used at sewage treatment plants. These are fixed parallel to one another at desired space on a rectangular steel frame and are called bar screens which are set in screen chamber. Now-a-days, these screens are generally kept at about 30-60° to the direction of flow so as to increase the opening area and to reduce the flow velocity and thus making the screen more effective. Screens can be either fixed or movable depending upon whether the screens are stationary or capable of motion. Fixed screens are permanently set in position while movable can be moved bodily for the purpose of cleaning.
c) Fine Screens - In this type of screen, spacing between bars is 1.5 mm to 3 mm size. It removes about 20% of suspending solids from sewage. These screens are made up of fine wire, brass or bronze plates with openings less than 1 cm. Metal used should be resistant to rust and corrosion. They may be disc or drum type and operated continuously by electric motors. These screens often get clogged and are to be cleaned frequently. They are, therefore, used for treating industrial wastewater or for municipal waste containing industrial wastewater. Cleaning frequency is governed by head loss through the screen. More the screen openings clogged more will be the head loss.
Key Takeaways
4.2.2 Sedimentation
The screens and the grit chambers remove most of the floating materials like paper, rags, cloth, wood and tree branches etc and the heavy inorganic settleable solids from the sewage. However, a part of the suspended organic solids which are too heavy to be removed as floating matters and too light to be removed by grit chambers are generally removed by the sedimentation tanks. This process is called sedimentation. The sedimentation tanks are thus designed to remove a part of the organic matter from the sewage effluent coming out from the grit chambers.
4.2.2.1 Theory of sedimentation
The settlement of a particle by gravity in liquid, when brought to rest, is opposed by the following factors:
1. Velocity of flow - It carries the particle horizontally. Greater the flow area, lesser is the velocity and hence more easily the particle will settle down.
2. Viscosity of water - In this particle travels. Viscosity is inversely proportional to temperature. Warm water is less viscous and therefore, offers less resistance to settlement.
3. Size, shape and specific gravity of the particle - Greater the specific gravity, more readily a particle will settle. Size and shape of the particle also affect the settling rate e.g. the weight and volume of a spherically shaped particle varies with the cube of its diameter and its area varies with the square of the diameter.
4. Sedimentation tanks - The clarification of sewage by the process of sedimentation can be affected by providing conditions under which the suspended material present in sewage can settle out. This is brought about in specially designed tanks called sedimentation tanks. Out of the three forces which control the settling tendencies of the particles, two forces i.e., the velocity of flow, and the shape and size of the particles, are tried to be controlled in these settling tanks. The third force i.e. viscosity of sewage or temperature of sewage is left uncontrolled, as the same is not practically possible.
4.2.2.2 Types of sedimentation tanks
Sedimentation tanks may function either intermittently or continuously. In the intermittent type of sedimentation tanks, sewage is stored for a certain period and kept under rest. After 24 hours, the settlement of suspended particles at the bottom of tank, the clear supernatant (i.e. cleaner sewage) from top is drawn off and the tank is cleaned. Tank is again filled with raw sewage and process is repeated. It is used in old days.
In continuous flow type of sedimentation tank, flow velocity is only reduced and sewage is not brought to complete rest. The velocity is sufficiently reduced by providing sufficient length of travel. Velocity is adjusted in such a way that the time taken by the particle to travel from one end to another is slightly more than the time required for settlement of that particle.
Sedimentation tanks may be rectangular or circular.
The detention time (t), for a rectangular tank = Volume of tank/Rate of flow = BLH/Q
Where,
B = Width of basin
H = Depth of water in the tank
Q = Discharge entering the basin
L = Length of basin
Detention time for a circular tank = D2 (0.011 L + 0.785 H)/Q
Where,
D = Diameter of tank
H = Vertical depth at wall or side water depth.
Detention time usually ranges between 4 to 8 hrs for plain sedimentation. The width of tank is normally kept equal to 10 m and not allowed to exceed 12 m. The length of the tank is not generally allowed to exceed 4 times the width. Cross-sectional area of the sedimentation tank is such as to provide a horizontal flow velocity ranging between 0.15 to 0.9 m/min, normally kept at about 0.3 m/min. The total amount of flow from the tank within 24 hrs generally equals the maximum daily demand of water.
Key Takeaways
4.2.3 Determination of settling velocity
Sedimentation is a natural process in which solids with higher density than the fluid in which they are suspended, settles under the action of gravity.
Detention Time = 4 to 8 hours
They are designed to reduce velocity and turbulence. It is of two types:
a) Plain Sedimentation
b) Sedimentation aided with coagulation
a) Plain sedimentation -Plain sedimentation is the process of removing suspended matters from the water by keeping it quiescent in tanks, so that suspended matter may settle down in the bottom due to force of gravity.
b) Sedimentation aided with coagulation - When this reaction occurs, the particles bind together, or coagulate (this process is sometimes also called flocculation). The larger particles, or floc, are heavy and quickly settle to the bottom of the water supply. This settling process is called sedimentation.
Settling velocity of particle in sedimentation tank is given by:
Vs = 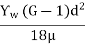
Where,
Vs = settling velocity of particle
Yw = density of water
G = specific gravity of water
µ = viscosity of water
d = particle size
Key Takeaways
4.2.4 Efficiency of ideal sedimentation tank
Hydraulic efficiency of settling tank is the flowing through period which can be expected to approach the critical detention period only in ideal tanks. Settling tanks efficiency is reduced by hr following currents:
Eddy currents - Set up by the inertia of the incoming fluid.
Surface currents – Induced due to wind in open tanks.
Vertical connection currents – It is due to thermal gradient along the depth of the tanks.
Density currents – It causes cold or heavy water to under run the basin and light water to flow across its surface.
The efficiency of the settling tank is dependent on the above mentioned currents, concentration of particles, shape of the basin and inlet and outlet arrangements.
Settling tanks should be capable of giving settled water having turbidity not extremely 20 JTU and preferable 5 JTU.
Key Takeaways
4.2.5 Design of settling tanks
4.2.5.1Settling
Solid liquid separation process in which a suspension is separated into two phases –
Type I: Discrete particle settling - Particles settle individually without interaction with neighboring particles.
Type II: Flocculent Particles – Flocculation causes the particles to increase in mass and settle at a faster rate.
Type III: Hindered or Zone settling –The mass of particles tends to settle as a unit with individual particles remaining in fixed positions with respect to each other.
Type IV: Compression – The concentration of particles is so high that sedimentation can only occur through compaction of the structure.
1) Vertical component2) Horizontal component: vh=Q/A
The path of the particle is given by the vector sum of horizontal velocity vh and vertical settling velocity vt.
All particles with Vt>Vo will be removed from suspension at some point along the settling zone.
The time to correspond to the retention time in the settling zone.
t= V/Q = LZoW/Q
Also, to= Zo/Vo
Therefore, Zo/Vo = LZoW/Q and Vo= Q/LW
or Vo= Q/As
4.2.5.5 Design Details
Circular: Diameter not greater than 60 m. generally 20 to 40 m.
4. Depth 2.5 to 5.0 m (3 m).
5. Surface Overflow Rate: For plain sedimentation 12000 to 18000 L/d/m2 tank area; for thoroughly flocculated water 24000 to 30000 L/d/m2 tank area.
6. Slopes: Rectangular 1% towards inlet and circular 8%.
Key Takeaways
4.2.6 Grit chamber
Grit removal is done in grit chambers, channels/basins. Inorganic solids such as pebbles, sand, silt eggshells, glass and metal fragments, heavier organics such as bone chips, seeds etc. when collected together, constitute grit, are removed from wastewater to prevent damage to pumps and to prevent their accumulation in sludge digester. Grit chambers are in fact sedimentation tanks designed to separate heavier inorganic by sedimentation due to gravitational forces and to pass forward the lighter organic material. It may be placed either after or before the screen.
Most of the substances in grit are abrasive in nature and will cause accelerated wear on pumps and sludge handling equipments. Grit deposits in areas of low hydraulic shear in pipes, sumps and clarifiers may absorb grease and solidify. These materials are not biodegradable and occupy valuable space in sludge digester so they should be separated from organic suspended solids.
Because, infiltration is a major source of inorganic, the quantity of grit varies with the type, age and condition of pipe in the collection system.
Grit removal facilities basically consist of an enlarged channel area where reduced flow velocities allow grit to settle out. Two configurations of grit chambers are available:
a) Channel type
b) Aerated rectangular basin
a) Channel type: - These are horizontal flow grit chambers in which the horizontal velocity is maintained at approximately 0.3 m/sec. Even a 25% increase in horizontal velocity may result in washout of grit whereas 25% decrease may result in retention of non-target organics. So, horizontal velocity must be artificially controlled. It is installed at small plants.
Grit from this type of grit chamber, may contain a sizable fraction of biodegradable organics that must be removed by washing or must be disposed off quickly to avoid nuisance problems. Grit containing organics must either be placed in a sanitary landfill or incinerated along with screenings to a sterile ash for disposal.
b) Aerated rectangular basin: - It is installed at large plants. In this, injection of compressed air creates turbulence and keeps lighter organic matter in suspension while the heavier grit falls to bottom. Here roll velocity is more important rather than horizontal velocity as it separates the non-target organics from the grit so artificial control of horizontal velocity is not required. Adjustment of air quantity provides settling control.
If sewage is anaerobic, aeration serves to strip noxious gases from liquid and to restore it to aerobic conditions which allows for better treatment. Aeration period is usually extended from 15 to 20 min.
Key Takeaways
4.2.7 Primary sedimentation and coagulation
When water contains fine clay and colloidal impurities which are electrically charged are continually in motion and never settle down due to gravitational force. Certain chemicals are added to the water so as to remove such impurities which are not removed by plain sedimentation. The chemical form insoluble, gelatinous, flocculent precipitate absorbs and entangles very fine suspended matter and colloidal impurities during its formation and descent through water. These coagulants further have an advantage of removing colour, odour and taste from the water. Turbidity of water reduced upto 5-10 ppm and bacteria
The following are the mostly used Coagulants with normal dose and PH values required for best floc formation as shown in Table
S.No. | Coagulant | PH Range | Dosage mg/l |
1. | Aluminum sulphate Al2(SO4)3, 18H2O | 5.5 – 8.0 | 5 – 85 |
2. | Sodium Aluminates, Na2Al2O4 | 5.5 – 8.0 | 3.4 – 34 |
3. | Ferric Chloride (FeCl3) | 5.5 – 11.0 | 8.5 – 51 |
4. | Ferric Sulphate Fe2(SO4)3 | 5.5 – 11.0 | 8.5 – 51 |
5. | Ferric Sulphate FeSO4.7H2O | 5.5 – 11.0 | 8.5 - 51 |
Removes upto 65%. Coagulants are chosen depending upon the PH of water. Alum or Aluminum sulphate is normally used in all treatment plants because of the low cost and ease of storage as solid crystals over long periods. The dosage of coagulants, which should be added to the water, depends upon kind of coagulant, turbidity of water, colour of water, PH of water, temperature of water and temperature of water and mixing & flocculation time. The optimum dose of coagulant required for a water treatment plant is determined by a Jar test as shown in Fig.1.
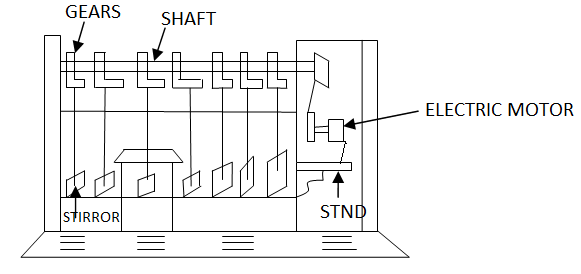
Fig.1: Jar Test
For starting the experiment first of all the sample of water is taken in every jar and added the coagulant in a jar in varying amounts. The quantity of coagulant added in each jar is noted. Then with the help of electric motor all the paddles are rotated at a speed of 30-40
r.p.m for about 10 minutes. After this the speed is reduced and paddles are rotated for about 20-30 minutes. The rotation of paddles is stopped and the floc formed in each Jar is noted and is allowed to settle. The dose of coagulant which gives the best floc is the optimum dose of coagulants.
The coagulants may be fed or allowed to enter either in powder form called dry feeding or in solution form called wet feeding. The mixing of coagulant with the water to form the floccs by the following methods.
Now a day’s some firms manufacture combined unit comprising of feeding, mixing, flocculator and clarifier device. The fig.2Shows used for sedimentation with coagulation
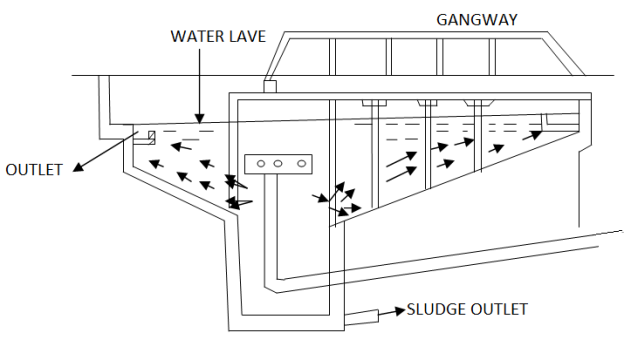
Fig.2: Sedimentation with Coagulation
4.2.7.1 Sedimentation with Coagulation
Water enters in this tank through central inlet pipe placed inside the deflector box. The deflector box deflects the water downwards and then it goes out through the holes provided sides of the deflector box. The water flows radially from the deflector box towards the circumference of the tank, where outlet is provided on the full periphery as shown in the Fig. All the suspended particles along with floc settle down on the sloppy floor and clear water goes through outlet. The sludge is removed by scrapper which continuously moves around the floor with very small velocity. Disinfection and repainting is to be carried out once in ayear before monsoon. Sludge pipes are to be flushed and kept clean. Bleaching powder may be used to control the growth of algae on the weirs. Scraper mechanism should be oiled and greased periodically.
4.2.7.2 Method of feeding coagulants
There are two methods of feeding coagulants to water
a) Dry Feeding: -Dry feeding is simple in operation and requires relatively less space for its working. The feeding machines are also cheaper. However, control of dose is difficult. In this case, the coagulant, in the powder form, is kept in the tank with hopper bottom.
The chemicals can be fed into water directly by dry feeding in the powder form. Alum being fairly fine and uniform in size can be fed easily by dry feeding. However, copperas and lime cannot be fed by dry feeding.
Dry feeding devices are generally in the form of a tank with hopper bottom. Agitating plates are placed inside the tank, so as to prevent arching of the coagulant. The coagulant in the powder form filled in the tank, and is allowed to fall in the mixing basin.
b) Wet Feeding: - The dosage in the wet feeding equipment can be adjusted more readily and can be easily controlled by means of automatic devices. However the chemicals which are corrosive in nature create problem in wet feeding.
4.2.7.3 Rapid Mixing
Rapid mixing after coagulant dosing is an import-tank design parameter. The coagulant must be uniformly mixed with the raw water. In case mixing is poor, local under- and overdosing occurs, resulting in poor performance of the process. In the flash mixer, coagulant chemicals are added to the water and the water is mixed quickly and violently to evenly distribute the chemicals through the water. Flash mixing typically lasts for 30 to 60 see. If the water is mixed for less than 30 seconds, then the chemicals will not be properly mixed into the water. However, if the water is mixed for more than 60 seconds, then the mixer blades will shear the newly forming floc back into small particles. After flash mixing, coagulation occurs.
Mixing is an important unit operation where one substance must be completely intermingled with another. In water treatment coagulant is mixed into water by rapid mixing. The process of dispersing chemical by mixing is known as rapid mix or flash mix.
Key Takeaways
4.3.1 Theory of filtration
The following are the mechanisms of filtration
2. Sedimentation – Absorption of colloidal and dissolved inorganic matter in the surface of sand grains in a thin film
3. Electrolytic action – The electrolytic charges on the surface of the sand particles, which opposite to that of charges of the impurities are responsible for binding them to sand particles.
4. Biological Action – Biological action due to the development of a film of microorganisms layer on the top of filter media, which absorb organic impurities.
4.3.1.1 Filtration is carries out in three types of filters
Key Takeaways
4.3.2 Hydraulics of filtration
Contaminated fluid causes most hydraulic system failures. Oil in a reservoir may look clean to the naked eye, but silt contamination particles too small to see can still wreck pumps, cause valves to stick, and erode cylinder bores. In many facilities, components may take the blame for problems in error, when contaminated fluid is the culprit. It is amazing that some plants will change pumps every six months (believing that is normal component life); when they could add a proper filtration system and get many times longer pump service life.
A well-filtered hydraulic system should not have particles in the fluid larger than 10 microns. (A micron is 0.000039 inches.) A contamination particle that measures 0.001 in. across is 25 microns. The smallest dirt particle that is visible to the naked eye is 40 microns. Simply looking at an oil sample is not a good way to tell if the filters are cleaning the fluid.
Nominal or absolute are common terms found in hydraulic filter micron ratings. A filter with a nominal rating takes out most of the particles that measure the same size or larger than the stated micron size. A filter with an absolute rating takes out all particles the same size or larger than the rated micron size. A newer filter-rating system called the beta ratio is replacing the old nominal and absolute designations.
The beta ratio indicates what size particles the filter removes, followed by the ratio of the number of this size particle in the fluid upstream from the filter, divided by number of particles that size in the fluid downstream from the filter. For example: a filter rating of beta 5 = 90 indicates the filter will remove 90 of every 100 particles of 5 micron or larger size from the fluid passing through it. The efficiency of this filter would be 98.9% -- or 100 - (100/90).
Most hydraulic filters employ a closely controlled paper fiber mat or a woven wire mesh element to trap particles. While woven wire is more expensive than paper, the ability to manufacture it with more precisely sized fluid flow openings makes it a better choice. Also, woven wire elements can withstand higher pressure drops without collapsing.
Key Takeaways
4.3.3 Slow sand
Slow sand filters are best suited for the filtration of water for small towns. The sand used for the filtration is specified by the effective size and uniformity coefficient
The effective size, D10, which is the sieve in millimeters that permits 10% sand by weight to pass. The uniformity coefficient is calculated by the ratio of D60 and D10.
4.3.3.1 Principles of Slow Sand Filtration
Key Takeaways
4.3.4 Rapid sand and pressure filters
Rapid sand filter are replacing the slow sand filters because of high rate of filtration ranging from 100 to 150m3/m2/day and small area of filter required. The main features of rapid sand filter are as follows.
4.3.4.1 Operation
The water from coagulation sedimentation tank enters the filter unit through inlet pipe and uniformly distributed on the whole sand bed. Water after passing through the sand bed is collected through the under-drainage system in the filtered water well. The outlet chamber in this filter is also equipped with filter rate controller. In the beginning the loss of head is very small. But as the bed gets clogged, the loss of head increases and the rate of filtration become very low. Therefore, the filter bed requires its washing.
Depth of sand - 60 to 75cm
Filter gravel - 2 to 50mm size
(Increase size towards bottom)
Depth of gravel -45cm
Depth of water over sand
During filtration - 1 to 2 m
Overall depth of filter
Including 0.5mfree board - 2.6m
Area of single filter unit - 100m2 in two parts of each 50m2 Loss of head - Max 1.8 to2.0m
Turbidity of filtered water - 1 NTU
Effective size of sand - 0.45 to 0.70mm
Uniformity coefficient of sand - 1.3 to 1
Pressure filter is type of rapid sand filter in closed water tight cylinder through which the water passes through the sand bed under pressure. All the operations of the filter are similar to rapid gravity filter; expect that the coagulated water is directly applied to the filter without mixing and flocculation. These filters are used for industrial plants but these are not economical on large scale.
Pressure filters may be vertical pressure filter and horizontal pressure filter. The Fig 3 shows vertical pressure filter. Backwash is carried by reversing the flow with values. The rate of flow is 120 to 300m3/m2/day.
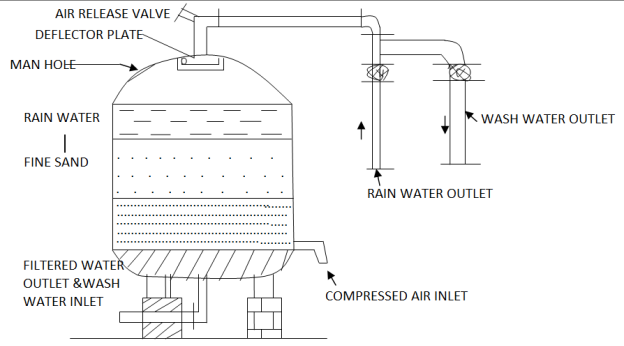
Fig.3: Pressure filter
4.3.4.2.1 Advantages
2. These are ideal for small estates and small waterworks.
3. These filters requires small area for installation.
4. Small number of fittings are required in these filters.
5. Filtered water comes out under pressure no further pumping is required.
6. No sedimentation and coagulant tanks are required with these units.
4.3.4.2.2 Disadvantages
3. The efficiency of removal of bacteria & turbidity is poor.
4. Change of filter media, gravel and repair of drainage system is difficult.
Key Takeaways
4.3.5 Backwashing
Key Takeaways
4.3.6 Design of slow and rapid sand filters.
4.3.6.1 Slow sand design filter design
Slow sand filter is made up of a top layer of fine sand of effective size 0.2 to 0.3mm and uniformity coefficient 2 to 3. The thickness of the layer may be 75 to 90 cm. Below the fine sand layer, a layer of coarse sand of such size whose voids do not permit the fine sand to pass through it. The thickness of this layer may be 30cm. The lowermost layer is a graded gravel of size 2 to 45mm and thickness is about 20 to 30cm. The gravel is laid in layers such that the smallest sizes are at the top. The gravel layer is retains for the coarse sand layer and is laid over the network of open jointed clay pipe or concrete pipes called under drainage. Water collected by the under drainage is passed into the out chamber
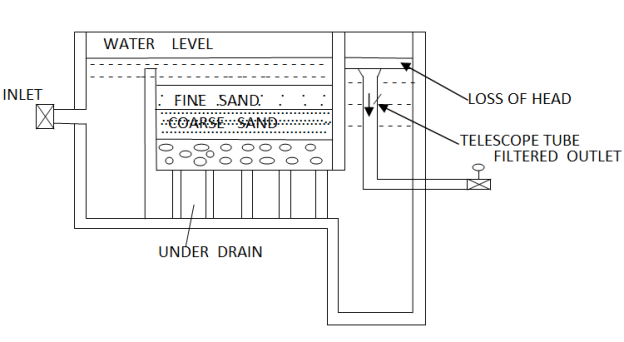
Fig.4: Slow sand filter
The water from sedimentation tanks enters the slow sand filter through a submersible inlet as shown in fig 4. This water is uniformly spread over a sand bed without causing any disturbances. The water passes through the filter media at an average at the rate of 2.4 to 3.6 m3/m2/day. This rate of filtration is continued until the difference between the water level on the filter and in the inlet chamber is slightly less than the depth of water above the sand. The difference of water above the sand bed and in the outlet chamber is called the loss of head.
During filtration as the filter media gets clogged due to the impurities, which stay in the pores, the resistance to the passage of water and loss of head also increases. When the loss of head reaches 60cm, filtration is stopped and about 2 to 3 cm from the top of bed is scrapped and replaced with clean sand before putting back into service to the filter. The scrapped sand is washed with the water, dried and stored for return to the filter at the time of the next washing. The filter can run for 6 to 8 weeks before it becomes necessary to replace the sand layer.
The slow sand filters are effective in removal of 98 to 99% of bacteria of raw water and completely all suspended impurities and turbidity is reduced to 1 N.T.U. Slow sand filters also removes odours, tastes and colours from the water but not pathogenic bacteria which requires disinfection to safeguard against water-borne diseases. The slow sand filters require large area for their construction and high initial cost for establishment. The rate of filtration is also very slow.
The algae growth on the overflow weir should be stopped. Rate of filtration should be maintained constant and free from fluctuation. Filter head indicator should be in good working condition. Trees around the plant should be controlled to avoid bird droppings on the filter bed, No coagulant should be used before slow sand filtration since the floc will clog the bed quickly.
4.3.6.2 Rapid sand design filter design
4.3.6.2.1 Backwashing of Rapid Sand Filter
The filter should be backwashed when the following conditions have been met:
4.3.6.2.3 Operational Troubles in Rapid Gravity Filters
2. Formation of Mud Balls
3. Cracking of Filters
4.3.6.2.4 Remedial Measures to Prevent Cracking of Filters and Formation of Mud Balls
Key Takeaways
4.4.1 Requirements of an ideal disinfectant
Key Takeaways
4.4.2 Various disinfectants
• Free Chlorine
• Monochloramine
• Ozone
• Chlorine Dioxide
• Iodine
• UV light
• Boiling (at household level in emergencies)
• Excess lime
• Silver
Key Takeaways
4.4.3 Chlorination and practices of chlorination
The use of chlorine has become universal in the disinfection of water.it is cheap reliable and no difficulty during handling.
The precise action by which chlorine kills bacteria in water is not known. Various theories have been put forward. A commonly accepted theory is the enzymatic hypothesis. According to this theory chlorine compounds formed when chlorine is added to water. When chlorine is dissolved in water at temperature 10°C to 100°C,it reacts to forms hypochlorous and hydrochloric acids within few seconds.
Cl2 + H2O = HCl + HClO
The hypochlorous acid, HOCL, ionizes into hydrogen ions and hypochlorite ions.
HOCL = H+ + OCL-
Thus, when chlorine is added into water 3 elements such as elemental chlorine, hypochlorous acid, hypochlorite ions are formed, and they remain in equilibrium at difficult concentrations.
4.4.3.1 Forms of application of chlorine
Chlorine may be applied to water in one of the following form.
1- Bleaching powder- also called calcium hypochlorite Ca (OCL) 2 is a chlorinated lime containing 33.5% of chlorine .it is therefore used for small installations or under emergency conditions. Commercial compounds such as HTH, pitticide, hoodchlor etc. are used instead of bleaching powder.
2- Chloramines- These compounds of ammonia and chlorine. In this test ammonia is added to water just before the chlorine is applied. The usual proportions are 1part of ammonia and 4.5 parts of chlorine by weight. The following reactions takes place-
H20 +CL2= HCL+HOCL
NH3+HOCL=H20+NH2CL (MONOCHLORAMINE)
NH2CL+HOCL=H20+NHCL2 (DICHLORAMINE)
NHCL2+HOCL=H20+NCL3 (TRICHLORAMINE)
3- Free chlorine-It is in gaseous or in liquid form. Gaseous chlorine is a greenish yellow poisonous substance with a typical odour and is 2.5 times heavier than air. Liquid chlorine is amber colored oily liquid and is about 1.44 times as heavy as water. When chlorine is subjected to a pressure of 7kg/cm2 then it is converted into liquid.
4- Chlorine dioxide- Its bacterial properties is greater than chlorine. It is unstable and it is produced at the point of use by passing the chlorine gas through sodium chlorite solution. The following reaction takes place.
2NACLO2+CL2=2NACL+2CLO2
4.4.3.2 Forms of chlorination
There are various methods of chlorination
1- Plain chlorination
2- Pre chlorination
3- Post chlorination
4- Double chlorination
5- Break point chlorination
6- Super chlorination
7- Dichlorination
1- Plain chlorination
It is application of chlorine which is applied to raw water supply as it enters the distribution system. It also includes the chlorination of raw water as it enters into reservoir to check the growth of weeds ,organic matter algae ,bacteria. It also removes the color and odour from water. The normal dose is between 0.5 ppm to 1 ppm.
2- Pre chlorination
It is application of chlorine to water before its treatment such as filtration, sometimes chlorination is done before raw water enters into sedimentation tanks. It reduces the quantity of coagulants required. It eliminates taste and odour.
3- Post chlorination-
It is the application of chlorine to water after its treatment. This is the standard form of chlorination in which chlorine is added to water as it leaved the slow sand filters or rapid filters before it enters the distribution system. The dose of chlorine is 0.1 to 0.2 ppm.
4- Double chlorination-
It is also called multiple chlorination. It is the application of chlorine in which chlorine is applied to just before water enters the sedimentation tanks and just it after leaves the filter plants. This is done specially when raw water is highly contaminated and contains large number of bacteria and other organic matter.
5- Break point chlorination-
When chlorine is added to water, it first reacts with ammonia. Breakpoint chlorination is the point where the demand for chlorine has been fully satisfied in terms of chlorine addition to water. When chlorine is added to water, a reaction is produced in the compounds present in the water. These compounds utilize the chlorine, resulting in zero chlorine residual. Once chlorine has been added to water, it is consumed by a type of chemical reaction that has a net effect of increased concentration of chlorine. For typical addition of chlorine, the rate of reaction instantly speeds up, reducing the concentration of chlorine. This is because chlorinated compounds acquire more chlorine.
The period wherein the concentration of chlorine goes into an upward slope is called the "breakpoint." In some cases, there can be no breakpoint seen because various organic compounds react at different rates.
Breakpoint chlorination is usually measured to determine when chlorination has been satisfied. This is a common practice in disinfecting water in industrial water systems as well as swimming pools. It is one of the most typical forms of chlorination where adequate chlorine is incorporated into the water to achieve the breakpoint, keeping the water well chlorinated and appropriate for its intended use.
6- Super chlorination-
Super chlorination is most commonly used when water has very high bacteria content and generally comes from river sources or where some form of pollution has occurred. It is also an important part of swimming pool maintenance because it keeps chlorine content at the right level to effectively kill off bacteria and other contaminants. Super chlorination is also known as shocking.
7- Dichlorination-
Dichlorination is the process of removing residual chlorine from disinfected wastewater prior to discharge into the environment. Sulphur dioxide is most commonly used for Dichlorination. Some Dichlorination alternatives include carbon adsorption, sodium metabisulfite, sodium bisulfite, and hydrogen peroxide. Sodium metabisulfite and sodium bisulfite are mainly used in small facilities because these materials are more difficult to control compared to sulfur dioxide.
Key Takeaways:
4.4.4 Water softening and ion-exchange process
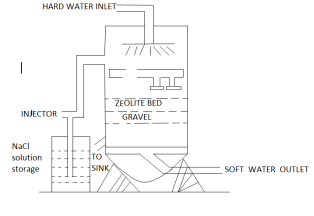
In the zeolite process it is also called ion exchange or Base Exchange method. In this process no such chemicals are added to water. Instead, hard waters are passed through a bed of a special material, loosely called zeolite which has no property of removing calcium and magnesium from water and substituting sodium in their place by ion exchange phenomenon. Zeolites are complex compound of aluminum, silica and soda, some forms are synthetic, and others are natural. Natural zeolite is mainly processed from green sand called glauconitic .it is green in color. The common artificial zeolite are called permutit. It has larger grains and color is white. Its chemical formula is 2sio2Al2o3Na2o.
For softening of water by zeolite process, hard water is percolated at a specified rate through a bed of zeolite. Zeolite holds sodium ion loosely and can be represented as Na2Z, where Z represents insoluble radical framework.
When the water passes through the zeolite the hardness causing ions (Ca2+, Mg2+ etc.) are retained by the zeolite as CaZ and MgZ respectively, while the outgoing water contains equivalent amount of sodium salts. The block diagram and
After some time, when the zeolite is completely changed into calcium and magnesium zeolites, then it gets exhausted (saturated with Ca2+ and Mg2+ ions) and it ceases to soften water. It can be regenerated and reused by treating it with a 10% brine (sodium chloride) solution.
CaZ + 2NaCl ® Na2Z + CaCl2
MgZ + 2NaCl ® Na2Z + MgCl2
4.4.4.1 Merits/ advantages of ion-exchange process
1- The process can be used to soften highly acidic or alkaline water.
2- It produces water of very low hardness (say 2ppm).
3- It is very good for treating water for use in high-pressure boiler.
4- It removes the hardness almost completely (about 10 ppm hardness only).
5- This process does not involve any type of precipitation, thus, no problem of sludge formation occurs
4.4.4.2 Demerits/disadvantages of ion-exchange process
1- The equipment is costly and more expensive chemicals are needed.
2- If water contains turbidity, then the output of the process is reduced.
3- Turbidity must be below 10 ppm. If it is more, it has to be removed first by coagulation and filtration.
4- This method only replaces Ca2+and Mg2+ ions by Na+ ions
5- High turbidity water cannot be softened efficiently by zeolite process
Key Takeaways
Ca(HCO3)2 + Na2Z ® CaZ + 2NaHCO3 Mg(HCO3)2 + Na2Z ® MgZ + 2NaHCO3 CaSO4 + Na2Z ® CaZ + Na2SO4 CaCl2 + Na2Z ® CaZ + 2NaCl MgSO4 + Na2Z ® MgZ + Na2SO4 MgCl2 + Na2Z ® MgZ + 2NaCl
|
|
References:
1. Manual on Water Supply and Treatment, C. P. H. E. E. O., Ministry of Urban Development,
Government of India, New Delhi
2. Manual on Sewerage and Sewage Treatment, C. P. H. E. E. O., Ministry of Urban
Development, Government of India, New Delhi
3. Steel and McGhee: Water Supply and Sewerage
4. Fair and Geyer: Water Supply and Wastewater Disposal
5. Hammer and Hammer Jr.: Water and Wastewater Technology
6. Raju: Water Supply and Wastewater Engineering
7. Rao: Textbook of Environmental Engineering
8. Davis and Cornwell: Introduction to Environmental Engineering
9. Kshirsagar: Water Supply and Treatment and Sewage Treatment Vol. I and II
10. Punmia: Water Supply and Wastewater Engineering Vol. I and II
11. Birdie: Water Supply and Sanitary Engineering
12. Ramalho: Introduction to Wastewater Treatment Processes
13. Davis Mackenzie L., Cornwell, David A., “Introduction to Environmental Engineering”
McGraw Hill Education (India) Pvt. Ltd., New Delhi.
14. Birdie: Water Supply and Sanitary Engineering
15. Ramalho: Introduction to Wastewater Treatment Processes
16. Parker: Wastewater Systems Engineering
17. A.K. Jain, Environmental Engineering, Khanna Publishing House