Module 4
Water Analysis
Hard water: is water that contains an required quantity of dissolved minerals (like calcium and magnesium) As rainwater falls, it is naturally soft. However, as water flows through the ground and into waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is essentially used as drinking water. Not only because of the health benefits, but also the flavour. Water that does not produce lather with soap readily is called hard water. Water hardness is usually measured as calcium hardness in milligrams per litre (mg/l) OR parts per million (ppm) OR in grains per gallon (GPG).
For eg: sea water, river water, spring water, lake water and well water.
Surface water hardly exceed hardness level above 200 mg/1 and softening is not at all required in most of the cases, unless the water is being polluted by some effluent sources. In case of groundwater, hardness level of more than 1000 mg/1 are quite common. Since, soft water is corrosive, therefore public water supply are usually not softened below 30 to 50 mg/1. The most accepted and commonly used water softening methods are cat ion exchange and precipitation method. In order to obtain maximum profit, the factors to be considered are a good choice of a softening process, quality of the raw water, the cost of softening chemicals and the cost of disposing of waste streams.
Precipitation methods
The principle that follows the precipitation method is to bind calcium cations Ca and magnesium cations Mg , with ions of CO3 and OH . The precipitate CaC03 and Mg (OH)2 formed are removed from the water. slake lime Ca(OH)2, Quick lime CaO , soda ash NaC03 and sodium hydroxide (caustic soda) NaOH, are reagents that are commonly used in water softening. Depending upon the quality of initial water, the following main precipitation methods are determined. a) Lime softening b) Lime - Soda softening c) Sodium Hydroxide softening Lime affects the carbonate hardness (alkalinity) and therefore can be used in order to decrease the carbonate hardness present in the initial water. This method however does not result in deep softening. Magnesium is removed from water if there is excess of OH” present. Water dissolved carbon dioxide is removed, total solids in the treated water diminishes and the total hardness in the lime treated water also reduces. But the pH increases to 10 or beyond. When lime is added to the hard water following reactions occurs, In the above reactions,
Lime Addition:-
Hardness Lime Precipitate
CO2 + Ca(OH)2 -- > CaCO3 + H2O Ca(HCO3)2 + Ca(OH)2 -- > 2CaCO3 + 2H2O Mg(HCO3)2 + Ca(OH)2 -- > CaCO3 + MgCO3 + 2H2O MgCO3 + Ca(OH)2 -- > CaCO3 + Mg(OH)2 CO2 the insoluble products do not contribute to the hardness, but It reacts with the lime, and thereby uses up some lime before the lime can start removing the hardness in water. Lime - Soda softening method is commonly practiced in most of the Public water supply. (Belan1984) The method is universal as water of almost any composition is treated with lime and soda. In this treatment, two reagents are used namely lime and soda ash. Lime as earlier discussed , decreases the carbonate hardness, (Mg2+) and removes C02 from the water.
Soda therefore reduces the non - carbonate hardness, mainly due to Ca2+, that shows after reaction with lime and the reaction occurs after the addition of soda ash is as follows.
Lime and Soda ash Addition:- Lime Precipitate MgSO4 + Ca(OH)2 -- > Mg(OH)2 + CaSO4 Soda ash Precipitate CaSO4 + Na2CO3 -- > CaCO3 + Na2SO4 2.
Ion Exchange Process
Ion exchange process to soften water ,using cations or anions .This is done by exchanging cations or anions with the calcium and magnesium ions in hard water. This process involves a reversible chemical reaction. However, we can use this technique only in dilute solutions. The equipment that we use for this purpose is ion exchangers.
Types
There are two types;
The materials used in cation exchangers include either weak acids or strong acids. Strong acid cation exchangers mainly contain sulfate functional groups. Weak acid cation exchangers mainly contain carboxyl groups. The materials that are used in anion exchangers include either weak bases or strong bases. Moreover, there are several categories of ion exchange process used for water softening, Dealkalization and demineralization. The ions that are part of the exchange process (the ions that exchange with the calcium and magnesium cations in hard water) include sodium ions, hydrogen cations, chloride anions and hydroxyl anions.
Zeolite process is a process of softening hard water thru ion exchange technique using a chemical compound zeolite. It possesses a chemical compound that has hydrated sodium aluminosilicate. Thus, the name of the process is called as zeolite process. Zeolite compound can exchange its sodium cations reversibly with calcium and magnesium ions in the water softening process.
There are two types of zeolite used in this process they include natural and synthetic zeolite. The natural form is found to be porous and synthetic form is a non-porous zeolite. however synthetic form possesses a high exchange capacity per unit weight than the natural form.
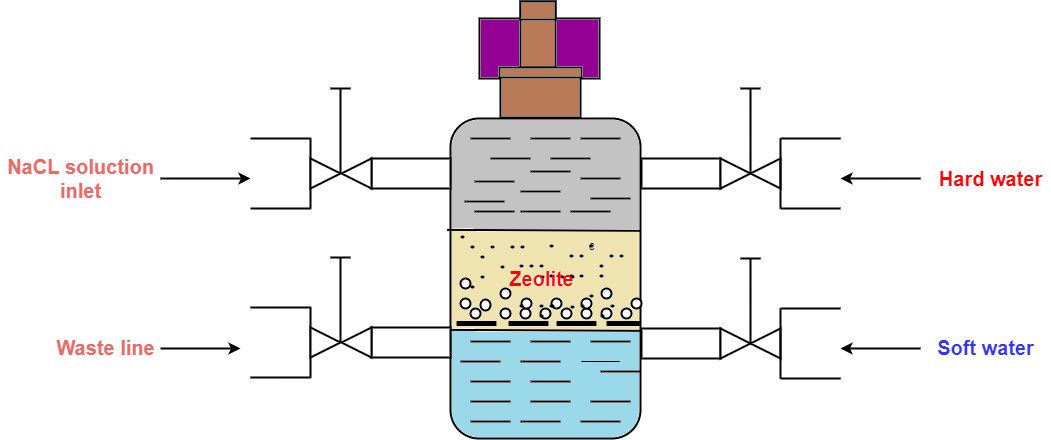
Figure 01: Cylinder Containing the Zeolite Bed
Process
In the water softening process, hard water is passed through a bed of zeolite (inside a cylinder) at a specified rate. Then the cations that cause the water-hardening will remain on the zeolite bed because these cations exchange with the sodium cations of zeolite. Therefore, the water coming out of this cylinder contains sodium cations rather than calcium and magnesium cations.
After some time, the zeolite bed gets exhausted, the water flow is stopped and treat the bed is treated with concentrated brine solution (10%) in order to regenerate the zeolite. The bed is treated with a brine solution, as it washes away all the calcium and magnesium ions, by exchanging them with sodium ions in a brine solution. hence, this treatment regenerates the zeolite.
Fuels :-
A fuel is the substance which on combustion produces a large amount of heart.
Classification of chemical fuels :-
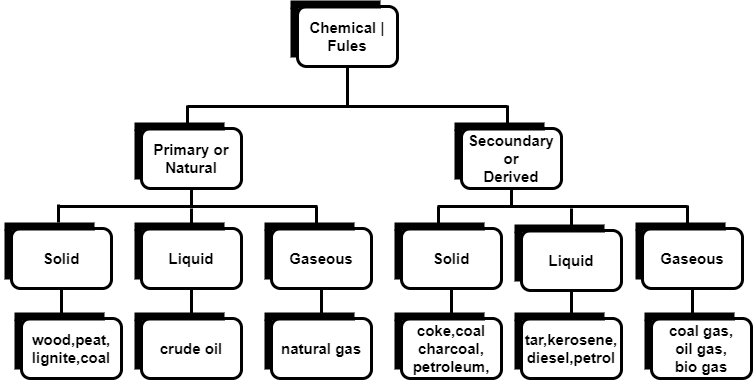
Chemical fuels are classified on the basis of occurrence into :-
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
Extra definitions :-
1] fuels :-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] chemical fuel :-
The fossil fuels , wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
Coal is mainly composed of C , h , N , S moisture volatile matter.
The purpose for coal analysis is
A sample of coal taken out from coal mine is analyzed in two ways.
Proximate analysis of coal ( 5 / 6 m )
Proximate analysis is the study or analysis of coal sample in which
A] moisture ( percent ) :-
Moisture percent = 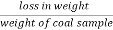 *100
*100
B] volatile matter (V.M) :-
Volatile matter (percent ) :- 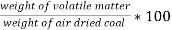
= 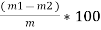
The volatile matter percent can be also determined by taking the fresh weight of the air dried coal but the loss in weight .at 925°c will be due to loss of moisture and volatile matter if W is the mass of coal left at 925° c heating,
Then ,
Volatile matter percent = 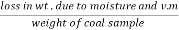 *100
*100
= 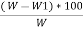 – moisture percent
– moisture percent
C] Ash percent :-
Therefore ,
Ash ( percent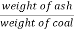 ) * 100 =
) * 100 = 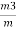 *100
*100
D] fixed carbon ( percent ) :-
It is found by calculation
f.c = 100 – ( moisture + v.m + ash )
significance ( important of proximate analysis )
Moisture :-
Volatile matter :-
However , the coals containing 15 – 25 percent of X.M on carbonization gives coke oven gas which is the source of various organic aromatic chemicals such coals have good caking property and caking the coals be obtained from the coals.
Overall , regarding burning of coal the coal the coal with lesser V.M is better quality coal.
Ash :-
Principle of ash :-
D ] fixed carbon :-
f.c( percent ) = 100 – ( moisture + V.M + ash )
Numerical :-
Given data :-
M=1.9 gm
( m – m1 )= 0.285 gm ( moisture loss )
Loss in weight due to moisture and VM = 0.36gm
Weight of coal = 2.15 gm
Weight of ash = 0.26gm
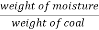
= 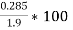 = 15 percent
= 15 percent
2. V.M ( percent ) :-
v.m = 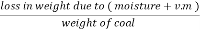 *10
*10
= 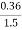 *100-15 = 24 – 15
*100-15 = 24 – 15
= 9 percent
3. Ash ( percent ) :-
Therefore ,
Ash = 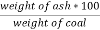
= 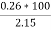
= 12 ( percent )
Principle :-
The C in the coal gets converted co2 and hydrogen to h2o vapors burning in the presence of pure o2
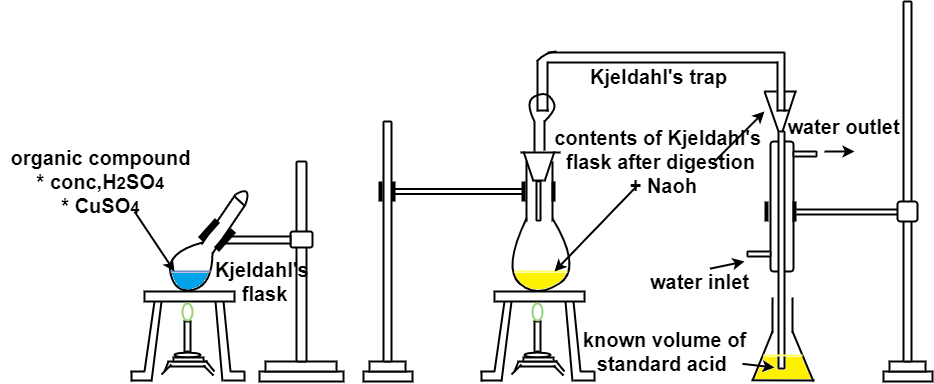
Ultimate analysis of coal :-
The analysis of coal in which percentage of C , N , H , S and O elements are found out is known as ultimate analysis .
A] C,H in coal :-
C + O2 – CO 2
2H + ½ - H2O
2KoH + CO2 – K2CAO3 + H2O
Cacl2 + 7H2O – Cacl2 – CacL2 .7h20
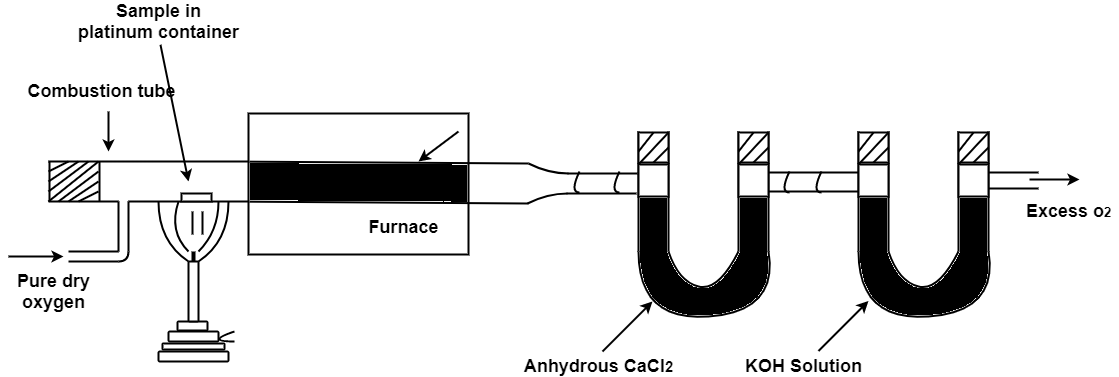
N ( percent ) – n in coal gets converted to anomnous sulphate by action of hot conc . H2SO4 and then on treatment with alkali solution .
C ( percent ) = 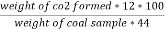
H ( percent ) = 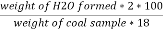
B] nitrogen in coal :-
The unused acid is determined by titration with NaOH solution.
N ( percent ) =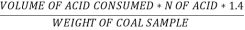
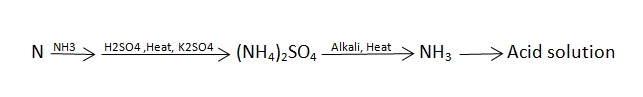
C] Sulphur in coal :-
S ( percent ) = 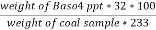
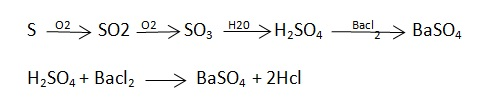
Importance of ultimate analysis:-
Carbon :-
Hydrogen :-
Nitrogen :-
Sulphur :-
Oxygen :-
Numerical :-
Given data :-
Increase in weight of any hydrous calcl2 = 0.55
Mass of coal = 0.75gms
Volume of 0.12 NHCL consumed by NH3 = 50 – 41
= 9 ml
Mass of coal = 1.8gms
Weight of Baso4 = 0.31 gm
Therefore ,
C percent ) = 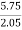 *
*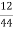 *100 = 76.5 percent .
*100 = 76.5 percent .
Increase in weight of any hydrous Cacl2
= weight of H2O formed
Mass of coal = 2.05gm
H ( percent ) = 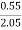 *
*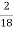 *100
*100
= 2.98 ( percent )
2. N ( percent ) = 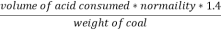
= 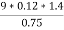
= 2.02 ( percent )
3. S ( percent ) = 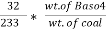 *100
*100
= 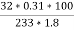
= 2.36 ( percent )
Theoretical calorific values
A] gouthal formula :-
Theoretically calorific value of coal can be calculated from proximate analysis data by using gouthal formula .
G.C.V Cal / gm = 82fc + a.VM
The values of constant are as under
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter .( if the liquid is volatile , then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction :- a bomb colorimeter consists of
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . the lid can be fitted air. Tight to bomb pot by screwing.
Calorimeter :-
Accessories :-
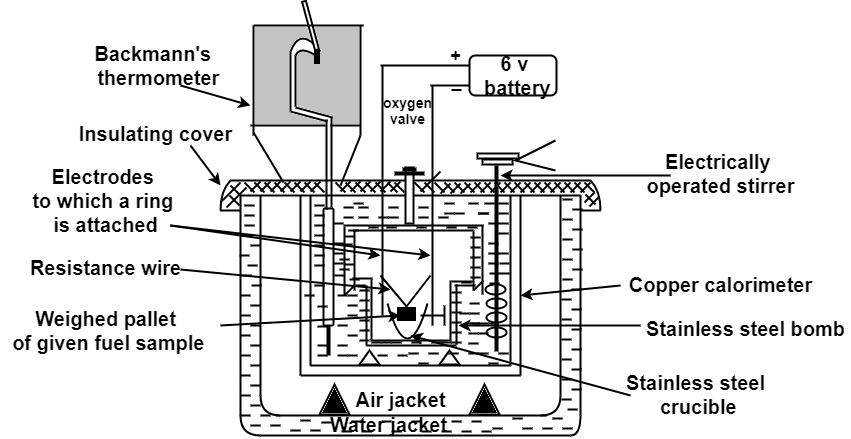
Fig: Bomb Caloriemeter
Working :-
Calculations :-
Gross calorific value of fuel = l calories / gm
Rise in temperature of water = ( t2 – t1 )
Heat liberated by burning fuel = heat absorbed by water and calorimeter
Therefore ,
XL = ( W+W) ( T2 – T1 )
G.CV = L = 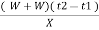 cal / gm
cal / gm
Note :-
Water equivalent of calorimeter – set ( w ) is determined by burning fuel of known gross calorific value and using the above equation.
Standard gross calorific values of some pure fuels are,
Naphthalene = 9622 Cal / gm
Benzoic acid = 6325 Cal / gm
Camphor = 9292 Cal / gm
Salicylic acid = 5269 Cal /gm
NCV for the fuel is calculated as below .
If H is percentage of hydrogen in the fuel, then the heat taken by water formed during combustion to convert it into steam is = 0.09h * 587 Cal / gm
And
NCV = GCV – 0.09h * 587 Cal / gm or kcal / kg.
Corrections :-
To get more accurate results , the following corrections should be considered.
Out of the total obtained little heat is given out by fuse wire when the current is passed for s – 10 sec .to start the combustion. Hence it must be subtracted. Are exothermic and the heat measured includes a small share by these acid formation.
2. Cooling correction ( te ) :-
If the time taken for water in calorimeter to cool from maximum temperature attained to the room. Temperature is t minutes and average cooling rate is dt / min , then the cooling correction to be added to rise in temperature is t.dt .
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
Principle :-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
3. Combustion chamber ( chimney ) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of it water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometer to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere .there are three holes for exhaust gas, water inlet and condensed steam.
Working :-
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
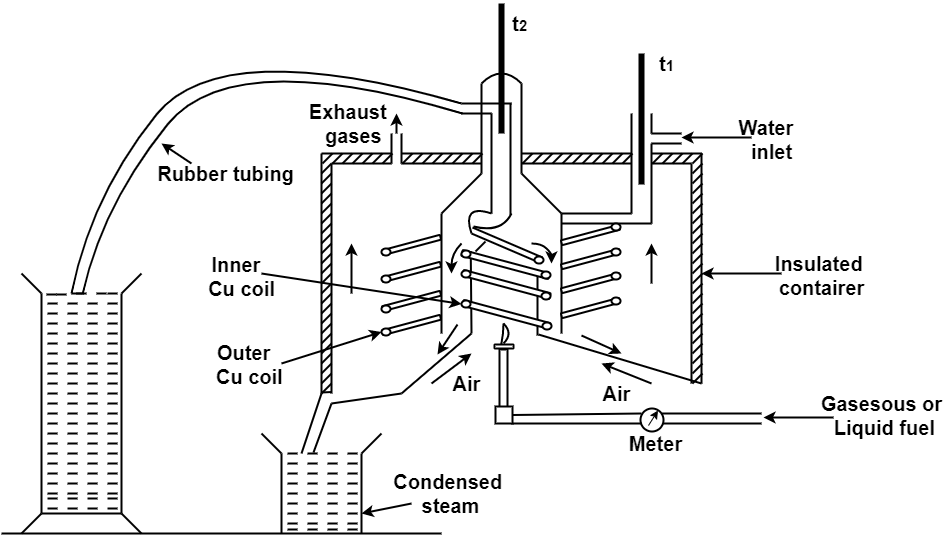
Fig: Boy’s gas calorimeter
Calculations :-
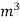
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 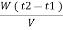 Kcal /
Kcal / 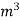
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
=  *587 kcal /
*587 kcal / 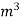
Therefore ,
NCV = GCV -  *587
*587
NCV = 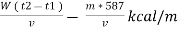
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
References:
1. University Chemistry By B.H. Mahan
2. University Chemistry By C.N.R. Rao
3. Organic Chemistry By I.L. Finar
4. Physical Chemistry By S. Glasstone
5. Engineering Chemistry By S.S. Dara
6. Polymer Chemistry ByFre W., Billmeyer
7. Engineering ChemistryBy Satya Prakash