Module 5
Polymer
Polymers are having larger molecular mass that is why they are mostly immiscible at a molecular level. The properties of the polymer blend are very much influenced by the structure obtained during the processing of their combination, and the way to study this structure is of key importance in materials science. The correlating up of the structural parameters to the processing and properties of a material and those studies are confirmed to be very important in the design of materials. Polymer blends are further categorized in three categories immiscible polymers blends, compatible polymer blends and miscible polymer blends. Immiscible polymer blends are those blends which exhibits more than two phases. This is the blend whose free energy of mixing is
ΔGm~ ΔHm>0
While the compatible polymer blends are miscible in a certain useful range of composition and temperature, but immiscible in others. Most compatible blends are immiscible and can be made compatible only by a variety of compatibilization techniques where as the miscible polymer blends homogenous down to the molecular level, which are associated with the negative value of free energy of mixture.
(polyhydroxy butyrate – hydroxyl Valente)
Biodegradation: -
It is a processes of converting polymer material into harmless simple gaseous products by the action on enzymes of microorganism and water.
A polymer which can be converted from harmless gaseous products by action of biological enzyme and water, is called as biodegradable polymers 3 components are important in the biodegradation of organic polymers.
A) Organisms: - like pseudomonas, bacillus, protozoa, acetobacter, various fungi, etc.
B) Environment: - suitable temp, moist condition, presence of salts, suitable ph.
C) Nature of polymer: -
Hydrophilic chain backbone of polymer contains O,N,3. atoms easily degrade.
3. Hydrophilic end groups
4. More amorphous nature of polymer
5. Small size of polymer material
Example: - PHBV
It is a co – polymer of hydroxy butyric acid and 3 hydroxyvaleric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.

STRUTURE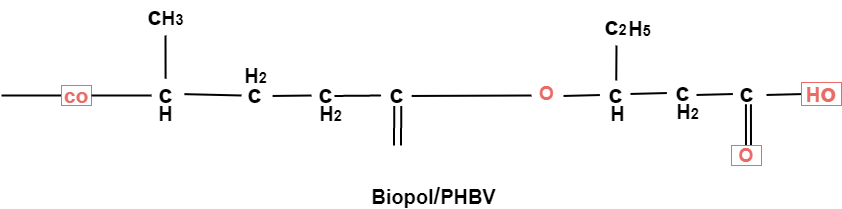
Properties: -
Limitations: -
Applications: -
The polymers have ability to conduct electricity are called as conducting polymers.
Structural requirements of polymer: -
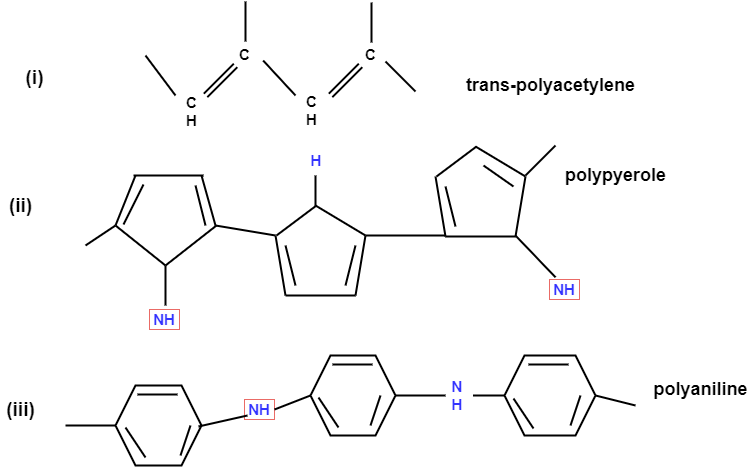
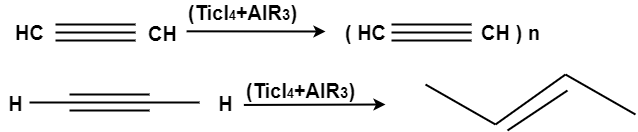
Poly acetylene: -
Structure: -
In doping process, polymer is either oxidized or reduced.
There are two type of doping
a] oxidative / p type doping
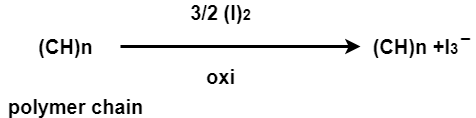
b] Reductive / n type doping

Applications: -
Preparation of Buna-S:
Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrine. Buna-S is the replacement of natural rubber while styrene, 2 monomers and butadiene play a major role in its derivation where as these 2 monomers is polymerized by two basically different process i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called as cold rubber. The obtained rubber is called as the Styrene Butadiene Rubber (SBR).
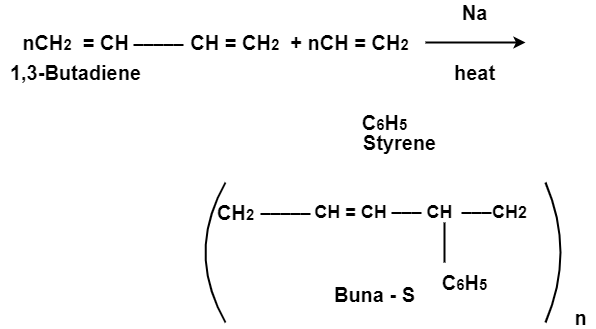
Applications:
(i) Buna-S is used as the natural rubber i.e., they are widely used in pneumatic tyres.
(ii) SBR is extensively used in coated papers.
(iii) They are highly used in building materials as a binding material.
(iv) SBR is also used as binder in lithium-ion battery electrodes.
Preparation of Buna-N:
Buna-N are commonly used as elastomers. They are unsaturated synthetic co-polymer. They are obtained by the co-polymerisation of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst. Nitrile rubber, NBR or Perbunan are another terms used for the Buna-N. They are synthetic rubber copolymer of acrylonitrile (ACN) and butadiene. Elastomers is natural or synthetic material which does not break when stretched and when released it return to its original length. The most important monomer used in preparing Buna-N is Acrylonitrile rubber which gives hardness, tensile strength, fuel and oil resistance. It usually contain 34% ACN. Grinding wheels can be made with nitrile rubber. When they are used for grinding smokes or fumes are emitted, which are known as acrid. The objectionable odor can be prevented if certain classes of diketones, which contain a conjugated system of two double bonds or unsaturated groups are added. Dibenzoylethylene, chloranil and anthraquinone help to prevent odor formation.
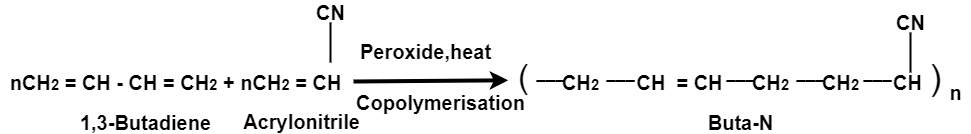
Buna-N are resistive to oil, fuel and other chemicals which means more Buna-N higher the resistance to oil but flexibility of the material is less. They are resistant to aliphatic hydrocarbons. They are non resistant to solvents but they may be attacked by ozone, ketones, esters and aldehydes.
Applications:
(i) They are used in the disposable non-latex rubbers, belts, gaskets.
(ii) They are used in preparation of adhesive and as a pigment binder.
Preparation of Neoprene:
Neoprene is primarily produced from petroleum by-products. They are the family of synthetic rubbers that are produced by polymerization of chloroprene. Neoprene has the ability to maintain stability at flexible temperatures. A good general-purpose rubber, neoprene is valued for its high tensile strength, resilience, oil and flame resistance, and resistance to degradation by oxygen and ozone; however, its high cost limits its use to special-properties applications. Neoprene referred as the rubber instead they are the plastics. They are made up of polychloroprene which was very first introduced. It has good chemical stability and maintains good flexibility over a wide range of temperature.
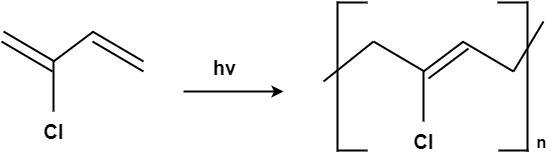
The original process of making neoprene consists of the usage of petroleum-based chemicals or oils. After that, it goes through a polymerization process. It transforms chemicals into rubber-type chips known as chloroprene. Consequently, we melt these chips and then we add foaming agents and thus it is formed.
Applications:
(i) They are used as the base for the adhesives, noise isolation in power transformations.
(ii) The burning point of neoprene is about 260o C.
(iii) They are used as the load bearing base.
(iv) They used in home accessories.
(v) Some experts evaluated that neoprene can be helpful in making the home based mask.
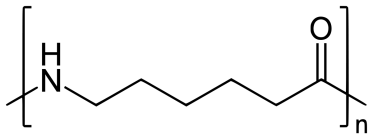
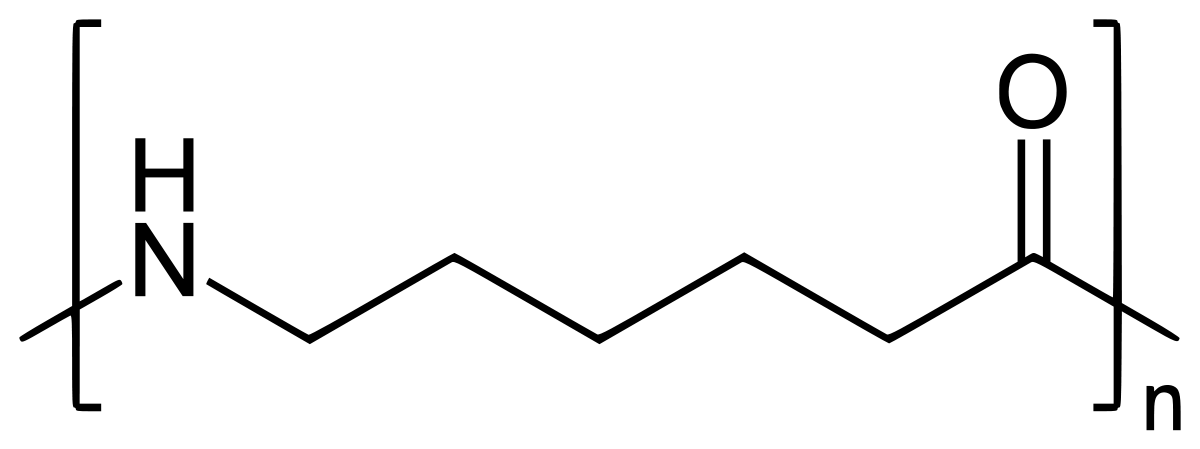
Preparation of Nylon-6:
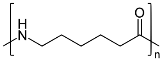
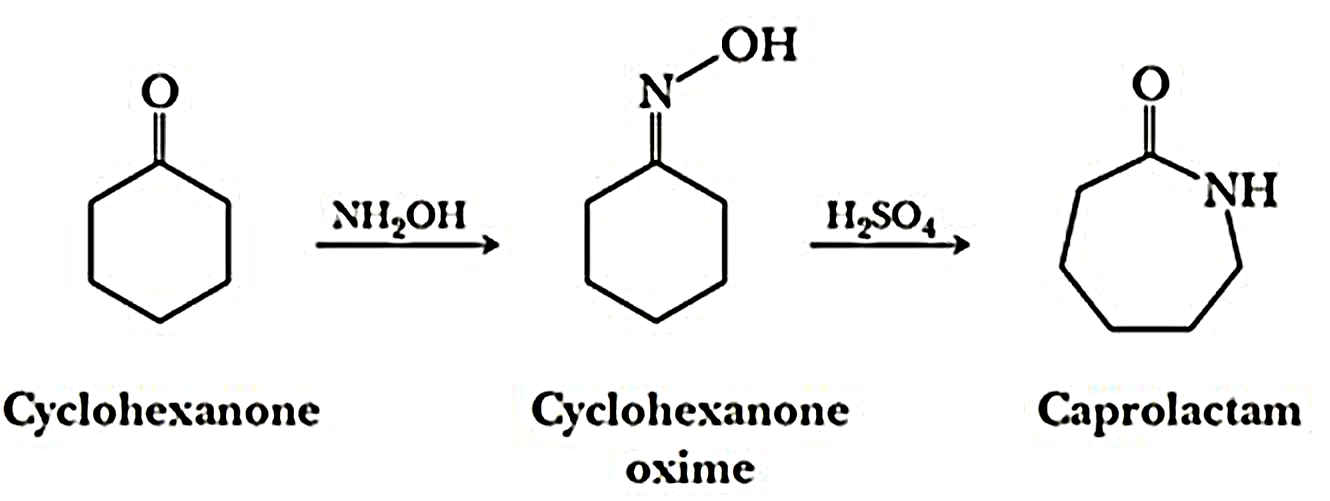
Nylon 6 is also called as the Polycaprolactam which is developed by Paul Schlack. They are semicrystalline polyamide.They have smooth surface and are featureless as a glass rods. Nylon 6 can be modified using comonomers or stabilizers during polymerization to introduce new chain end or functional groups, which changes the reactivity and chemical properties. It's often done to change its dyeability or flame retardance. Nylon 6 is synthesized by ring-opening polymerization of caprolactam. When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4–5 hours, the ring breaks and undergoes polymerization. Then the molten mass is passed through spinnerets to form fibres of nylon 6.
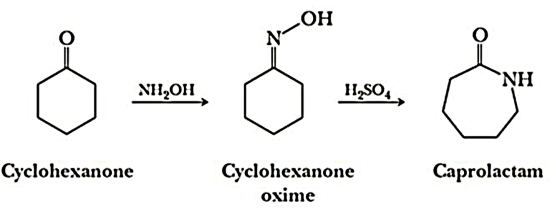
On further heating of Caprolactam at 533K with inert atmosphere of nitrogen for about 4-5 hours it gives
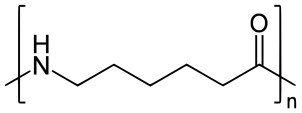
(Nylon-6)
Application:
(i) Nylon 6 is widely used for gears, fittings, bearings and automotive industry for various parts and as a material for power tools and housings.
(ii) It is used as threads in bristles for toothbrushes, surgical sutures and strings for acoustic and classical instruments.
(iii) It is used in production of large variety of ropes, filaments, nets as well as knitted garments.
(iv) It can also be used in gun frames, such as those used by Glock, which are made with composite of Nylon 6 and other polymers.
Preparation of Nylon 6,6:
Nylon 6,6 is a polyamide made from adipic acid and hexamethylenediamine by polycondensation. The resulting polymer is extruded into a wide range of fiber types. The fiber are drawn and stretch in a process that increases their length and reorients the material molecules parallel to one another to produce a strong elastic filament. The thermo plasticity of nylon permits permanent cripping of the fibres and provides bulk and stretch properties. The Nylon 6,6 is prepared from Nylon salt i.e., prepared by reacting the hexamethylene diamine and adipic acid in boiling methanol. The comparatively insoluble salt precipitates out from methanol. A 60% of aqueous solution of salt is then run into a stainless steel autoclave together with a trace of acetic acid to limit the molecular weight. The vessel is sealed and purged with oxygen free nitrogen and the temperature raised to 220oC at about the pressure of 1-7 MPa is developed. After 1-2 hours the temperature is raised to 270-280oC and steam blend off to maintain the pressure.
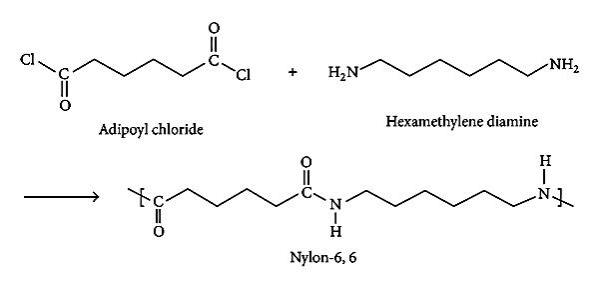
Preparations of Monomers:
Preparations of Terylene:
Terylene is a polyester fibre formed by the polymerisation of dimethyl terephthalate with ethylene glycol. Dimethyl terephthalate is heated with ethylene glycol at 503 K in the presence of zinc acetate and antimony trioxide as a catalyst when dihydroxydiethyl terephthalate (monomer) is formed, Dihyd- roxydiethyl terephthalate on heating polymerises to give terylene.
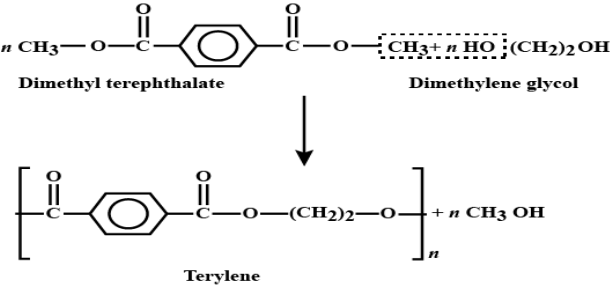
Applications:
(i) Terylene are very strong fiber and suffer very little loss in strength when wet.
(ii) They are elastic in nature that is why they possess the property of resist creasing.
(iii) It can be set into permanent pleats when subjected to the correct temperature at time of manufacturing.
(iv) They are easily washable and quickly dry.
(v) It is non-injured by acidic substances when used in the moderation process.
Grignard reagent is widely is used as organmetallic reagents. They are prepared in ethereal solution by the reaction of organic halide with magnesium.
 RX + Mg RMgX
RX + Mg RMgX
Where, X are halides i.e., Chlorine, bromine, Iodine
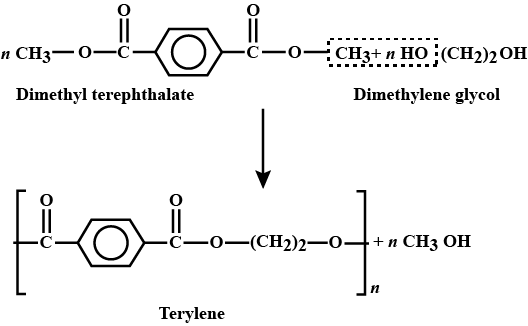
They are quite reactive in general while stable in ethereal solution. Where as the organometallic reagents are those compounds which contains carbon-metal bonds.
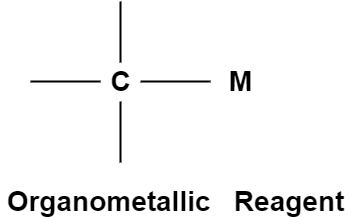
The compound attached to the organic compound may be the Li or Mg i.e., organolithium reagent while in the presence of Magnesium this is called as the Grignard Reagent. The Grignard Reagent play as the most versatile source of nucleophilic carbon. The nucleophilic character of organometallic reagents stems from the fact that the C-M bond is polarized in such a way that the carbon atom is negative while the metal atom is positive.
In the above figure the carbon metal has ionic character. Carbon is not a very electronegative element instead it is very reactive when it bears a negative charge. They are prepared before they use as because they are so reactive. Organolithium reagents are available commercially as solutions in the inert solvents such as diethyl ether, tetrahydrofuran (THF) or pentane. They must be handledunder inert atmosphere.
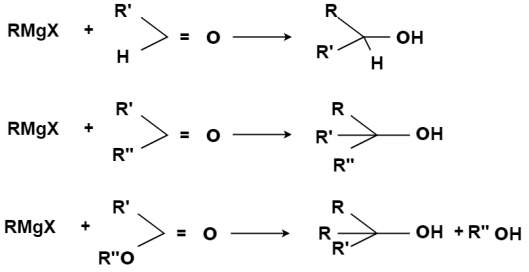
With Carbonyl Compounds:
Organometallic Reagents as Bases:
Organometallic reagents such as phenylmagnesium bromide and methyl lithium are the strongest bases. They will deprotonate compounds such as amines, alcohol and carboxylic acid.
Organometallic Reagents as Nucleophiles:
An organometallic reagents are base or nucleophile it directly depends upon its bond formation whether with carbon or hydrogen atom. If a reactant contains an electrophilic carbon and does not contain O-H or N-H groups, then an organometallic reagent will act as a nucleophile towards that electrophilic carbon atom. Carbonyl group is most common source of electrophilic carbon, majorly the carbonyl group of aldehydes or ketones.
A fundamental principle that guides the development of logical approaches to the preparation of new molecules is that carbon-carbon bond formation requires the interaction of molecules which contain carbon atoms of opposite polarity.
Applications:
Grignard reaction is used in the alkylation of aldehydes and ketones.
References:
1. University Chemistry By B.H. Mahan
2. University Chemistry By C.N.R. Rao
3. Organic Chemistry By I.L. Finar
4. Physical Chemistry By S. Glasstone
5. Engineering Chemistry By S.S. Dara
6. Polymer Chemistry ByFre W., Billmeyer
7. Engineering ChemistryBy Satya Prakash