UNIT-3
Electrochemistry
This equation is named after the name of scientist who discovered is Walther Nernst. Nernst equation plays a major role in relating the Reduction Potential with the electrode potential, temperature of the chemicals which are undergoing the oxidation or reduction.(Reduction Potential is used to measure the tendency of the chemical species to acquire or loose electron to an electrode.)
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation ………..(i)
 G=-nFE ………..(ii)
G=-nFE ………..(ii)
Where,
n=no. of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE* ………….(iii)
G*=-nFE* ………….(iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ ………….(iv)
G= G*+RT lnQ ………….(iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ …………….(v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6) ……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q …….(vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q ……..(viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K …….(ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Applications:
1- This is used in the solubility product and potentio-metric titration.
2- It is used to calculate the potential of ion charge.
3- It is used in oxygen and aquatic environment.
Lead storage battery is the most common device used to store energy in the portable form. This is also called as lead acid battery. Although the batteries are reliable, which contain acidic material inside that required a proper disposal method after its complete use. These batteries have moderate power density and good time. The battery consists of lead grids on its electrodes. The anodic grid opening is filled with spongy lead while the cathodic grid consists of lead oxide (PbO2).
Charge Chemistry of the battery:
Charge batteries are those batteries which can be recharged after single use. In this type of battery each plate contain negative as well as the positive end. The negative plate is of lead while the positive plate is made up of lead oxide in an electrolyte of approx 4.0M sulphuric acid.
Negative plate reaction:
PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO4–(aq)
Positive plate reaction:
PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e–
Combining these two reactions, the overall reaction is the reverse of the discharge reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq)
Discharge Chemistry of the Battery:
The positive and negative plate of the batteries becomes lead sulphate. Due to the loss of sulfuric acid from electrolytes it becomes the water.
Negative plate reaction:
Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2e–
Positive plate reaction:
PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Combining these two reactions, one can determine the overall reaction:
Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq) → 2PbSO4(s) + 2H2O(l)
Corrosion: Corrosion is defined as the phenomenon in which metal get a coated covering over its whole body due to the chemical reaction from its surrounding that result in the conversion of metal into the oxide, salt or any other compound. In common language this process is called as the rusting of iron. The theories behind the corrosion of the metal are:
1-Direct Corrosion
2-Electro-Chemical Corrosion
3-High-Temperature Oxidation
Direct Corrosion:
The Direct Corrosion is the simplest corrosion which occurs directly in the environment temperature. Under this there is a chemical attack which includes oxidation under which the oxygen present in the environment combines with all the possible part of the surface of material.
Fe + O + 2CO2 + H2O → Fe(HCO3) 2
2Fe(HCO3)2 + O → 2Fe(OH)CO3 + 2CO2 + H2O
Fe(OH)CO3 + H2O → Fe(OH)3 + CO2
Electro-Chemical Corrosion:
The corrosion takes place due to chemical reaction in combination with electrolysis. It takes place at room temperature when metal come in contact with the moisture. The presence of electrolyte, current must be passing through the circuit and most important circuit must be closed for this type of corrosion.
High-Temperature Oxidation:
The formation of scales and oxides in iron rusting took place for rusting of ferrous alloys at high temperature while the other form of the corrosion may be noted when liquid metal flow through other metals. The corrosion is caused due to the tendency of the solid to dissolve into the liquid metal unto the limit of solubility.
Causes:
1- Corrosion took place due to the presence of salt.
2- Corrosion took place due to the inadequate design procedure.
3- Presence of excess water-cement ratio causes corrosion.
4- Internal structure of metal.
Prevention:
1- Maintaining a high degree of workmanship.
2- Use of high quality and impermeable concrete.
3- Using the correct cement-water ratio.
The homogenous and physical part of the system is bounded by any surface which is mechanically separable is termed as phase. The phase may be the in the gaseous form, solid form or liquid form. The boundary present between the two interfaces is called as the interface. Air constitutes a single phase only as it contains a mixture of nitrogen, oxygen, carbon dioxide, water vapour etc. A system consisting of only one phase is said to be homogeneous. A mixture of two immiscible liquids such as water and benzene, will exist in two distinct liquid phases and in addition there will be a vapour phase. Thus there will be three phases each separated from the other by a well-defined bounding surface while the system consist of more than one phase is called as the heterogenous phase.
Phase Rule: If the equilibrium between any number of phases is not influenced by gravity, or electrical by surface action but are influenced only by temperature, pressure and concentration , then the number of degrees of freedom (F) of the system is related to the number of components (C ) and number of phases (P) by the following phase rule equation:
F=C-P+2
Liquid Phase:
(i) Liquid phase is depend on the number of liquids present and their missibility.
(ii) 2 miscible liquids form 2 separate liquid phase.
(iii) 2 miscible liquids form 1 liquid phase only.
Solid Phase:
Each solid forms a separate phase while number of solid phase depends on the number of solids present in it.
Gaseous Phase:
Due to the thoroughly miscible proportions of gases they form phases only.
Eg.: N2 and H2 mixture form phases only.
Applications to water system: In water there is only one component i.e., water and its phases: ice, water, steam that is solid, liquid and gaseous form. 

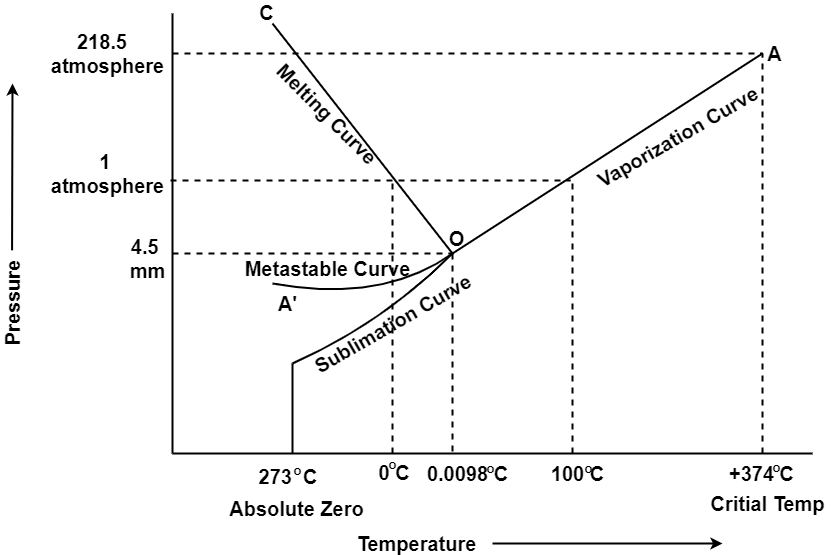
In the figure given above, the horizontal movement leads to be responsible for temperature change while the vertical curve responsible for the pressure change. In the above figure there are three curves i.e., OA,OB,OC which represents the equilibrium conditions between two phases solid with vapour, vapour with liquid and liquid with solid phase of water. Curve OC represents the equilibrium between solid and liquid phase of water. The curve is known as the fusion pressure or melting point curve. Along this curve there are two phases in equilibrium that is ice and water. At atmospheric pressure ice and water can be in equilibrium only at one temperature i.e, the freezing point of the water.
Here, C=1,
P=2
F=C-P+2
=1
Curve OB represent the equilibrium between liquid and vapor. This is called as the vaporization curve. Here also it is necessary to state either temperature or pressure. Eg.: at atmospheric pressure water and vapor can exists in equilibrium only at 1 temperature i.e., the boiling point of the water. Water vapor has also one degree of freedom.
F=C-P+2
=1
References:
1. University Chemistry By B.H. Mahan
2. University Chemistry By C.N.R. Rao
3. Organic Chemistry By I.L. Finar
4. Physical Chemistry By S. Glasstone
5. Engineering Chemistry By S.S. Dara
6. Polymer Chemistry ByFre W., Billmeyer
7. Engineering ChemistryBy Satya Prakash