Unit-5
Chemical and electrochemical energy sources
Fuels:
Fuel is the substance which on combustion produces a large amount of heat. Fuel is a combustible substance, containing carbon as a main constituent, which on proper burning gives large amount of heat, which can be used economically for domestic and industrial purpose. E.g.: Wood, charcoal, coal, kerosene, petrol, diesel, producer gas, oil gas etc.
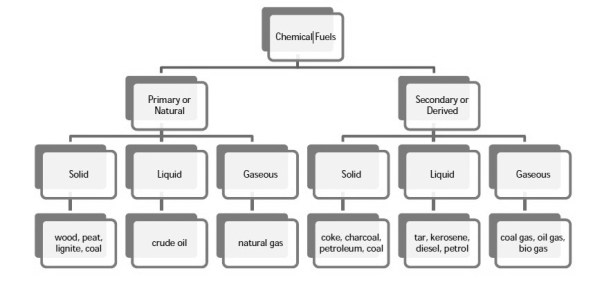
Classification of chemical fuels:
Chemical fuels are classified based on occurrence into:
- Primary (natural)
- Secondary (derived) types / manmade.
The secondary fuels are obtained from primary fuel by processing or they are manmade. e. g.: charcoal is obtained from wood by partial combustion of wood, ethyl alcohol is obtained by fermentation of carbohydrates.
Both the primary and secondary fuels are further classified based on physical state into solid liquid and gaseous fuels.
Key Takeaways:
1) Fuel is the substance which on combustion produces a large amount of heat.
2) The secondary fuels are obtained from primary fuel by processing or they are manmade.
5. 2 Classification of chemical energy (fuels)
|
- The fuel should be easily available.
- It should be dry and should have less moisture content.
- Dry fuel increases its calorific value.
- It should be cheap, easily transportable, and has high calorific value.
- It must have moderate ignition temperature and should leave less ash after combustion.
- The combustion speed of good fuel should be moderate.
- It should not burn spontaneously to avoid fire hazards.
- Its handling should be easy and should not give poisonous gases after combustion.
- The combustion of good fuel should not be explosive.
The gross calorific value of solid fuels and liquid fuels can be determined by a bomb calorimeter. (if the liquid is volatile, then it is filled in a polythene capsule of negligible mass then used in the experiment.
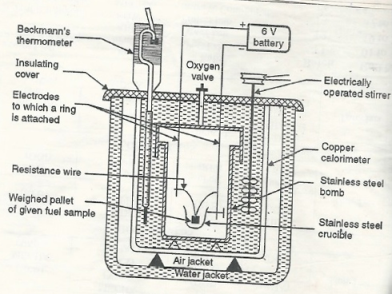
Construction: a bomb calorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot:
It is a cylindrical strong stainless-steel pot having a lid. The lid can be fitted with air. Tight to bomb pot by screwing.
- There are two types of electrodes fitted through the lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel. There is a thin resistance wire tied to the electrodes in a loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter:
- There is a stainless steel or copper calorimeter in which the bomb pot is kept. It contains a known volume of water and the water is kept circulating in the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter, which can record the rise in temperature of welter due to absorbing in a heat generated.
- There are insulator stands between the calorimeter and water jacket.
Accessories:
- There is a pellet press to convert the powder of solid fuel to pellet form. For a liquid fuel, a capsule of negligible weight can be used.
- There is an oxygen cylinder with a pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D. C battery of about 6 volts to start the combustion of fuel.
|
Fig: Bomb Calorie meter
Working:
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible. Keep the crucible in the ring of the electrode. Keeps the resistance wire touching the fuel.
- Add about 10 ml of distilled water at the bottom of the bomb pot and fix the lid tightly to the bomb by screwing.
- Fill the bomb with oxygen at a pressure of about 25 kg / cm².
- Place the bomb in the calorimeter add a known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs. Keep the thermometer and stirrer in the water of the calorimeter.
- Put the plastic cover on the end make electrical connections from the battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water (t1° c).
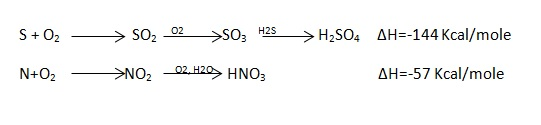
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5. These gases get dissolved in the distilled water in a bomb to form H2SO4 and HNO3 (along with liberation little heat).
- Note the maximum temperature reached. After that note, the rate of fall of temperature per minute and the time is taken for reaching to initial temp are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Calculations:
Gross calorific value of fuel = l calories / gm The rise in temperature of water = ( t2 – t1 ) The heat liberated by burning fuel = heat absorbed by water and calorimeter
Therefore, XL = (W+W) (T2 – T1) G. CV = L = W+W t2-t1 Xcal/gm
Note: Water equivalent of calorimeter – set (w) is determined by burning fuel of known gross calorific value and using the above equation. Standard gross calorific values of some pure fuels are, Naphthalene = 9622 Cal / gm Benzoic acid = 6325 Cal / gm Camphor = 9292 Cal / gm Salicylic acid = 5269 Cal /gm
NCV for the fuel is calculated as below. If H is the percentage of hydrogen in the fuel, then the heat taken by water formed during combustion to convert it into steam is = 0. 09h * 587 Cal/gm And NCV = GCV – 0. 09h * 587 Cal / gm or kcal / kg.
Corrections: To get more accurate results, the following corrections should be considered.
Out of the total obtained little heat is given out by fuse wire when the current is passed for s – 10 sec. to start the combustion. Hence it must be subtracted. Are exothermic and the heat measured includes a small share by these acid formations.
2. Cooling correction (te): If the time taken for water in the calorimeter to cool from maximum temperature attained to the room. Temperature is t minutes and average cooling rate is dt / min, then the cooling correction to be added to rise in temperature is t. dt.
This calorimeter is used to measure the calorific value of gaseous fuels and highly volatile fuels. |
Key Takeaways:
1) The gross calorific value of solid fuels and liquid fuels can be determined by a bomb calorimeter.
2) Bomb pot is a cylindrical strong stainless-steel pot having a lid.
3) The calorimeter is used to measure the calorific value of gaseous fuels and highly volatile fuels.
Coal is mainly composed of C, h, N, S moisture volatile matter. The purpose of coal analysis is
A sample of coal taken out from coal mines is analyzed in two ways.
Proximate analysis of coal ( 5 / 6 m ) Proximate analysis is the study or analysis of a coal sample in which
A] moisture (percent):
Moisture percent = loss in weight of coal sample*100
B] volatile matter (V. M):
Volatile matter (percent): weight of volatile matter weight of air-dried coal *100 = (m1-m2)m*100
The volatile matter percent can be also determined by taking the fresh weight of the air-dried coal but the loss in weight. at 925°c will be due to loss of moisture and volatile matter if W is the mass of coal left at 925° c heating, Then, Volatile matter percent = loss in wt. due to moisture and v. mweight of coal sample *100 = W-W1*100W – moisture percent
C] Ash percent:
Therefore, Ash (percent weight of ash weight of coal) * 100 = m3m*100
D] fixed carbon (percent): It is found by calculation f. c = 100 – (moisture + v. m + ash)
significance (important of proximate analysis)
Moisture:
Volatile matter:
However, the coals containing 15 – 25 percent of X. M on carbonization gives coke oven gas which is the source of various organic aromatic chemicals such coals have a good caking property and caking the coals be obtained from the coals. Overall, regarding the burning of coal, the coal with lesser V. M is better quality coal.
Ash:
Principle of ash:
D] Fixed carbon:
f. c(percent) = 100 – (moisture + V. M + ash) |
There are two methods to analyze coal: ultimate analysis and proximate analysis. The ultimate analysis determines all coal component elements, solid or gaseous and the proximate analysis determines only the fixed carbon, volatile matter, and moisture and ash percentages. The ultimate analysis is determined in a properly equipped laboratory by a skilled chemist, while proximate analysis can be determined with a simple apparatus.
- Measurement of moisture: The determination of moisture content is carried out by placing a sample of powdered raw coal of size 200- micron size in an uncovered crucible, which is placed in the oven kept at 108 +2 °C along with the lid. Then the sample is cooled to room temperature and weighed again. The loss in weight represents moisture.
- Measurement of the volatile matter: A fresh sample of crushed coal is weighed, placed in a covered crucible, and heated in a furnace at 900 + 15 oC. The sample is cooled and weighed. Loss of weight represents moisture and volatile matter. The remainder is coke (fixed carbon and ash).
- Measurement of carbon and ash: The cover from the crucible used in the last test is removed and the crucible is heated over the Bunsen burner until all the carbon is burned. The residue is weighed, which is the incombustible ash. The difference in weight from the previous weighing is the fixed carbon. In actual practice Fixed Carbon or FC is derived by subtracting from 100 the value of moisture, volatile matter, and ash.
- Proximate analysis: The proximate analysis indicates the percentage by weight of fixed carbon, volatiles, ash, and moisture content in coal. The amounts of fixed carbon and volatile combustible matter directly contribute to the heating value of coal. Fixed carbon acts as the main heat generator during burning. High volatile matter content indicates easy ignition of fuel. The ash content is important in the design of the furnace grate, combustion volume, pollution control equipment, and ash handling systems of a furnace.
Fixed carbon: Fixed carbon is the solid fuel left in the furnace after the volatile matter is distilled off. It consists mostly of carbon but also contains some hydrogen, oxygen, sulfur, and nitrogen not driven off with the gases. Fixed carbon gives a rough estimate of the heating value of coal.
Key Takeaways:
1) The ultimate analysis determines all coal component elements, solid or gaseous and the proximate analysis determines only the fixed carbon, volatile matter, and moisture and ash percentages.
2) The ultimate analysis is determined in a properly equipped laboratory by a skilled chemist, while proximate analysis can be determined with a simple apparatus.
3) The proximate analysis indicates the percentage by weight of fixed carbon, volatiles, ash, and moisture content in coal.
4) The ash content is important in the design of the furnace grate, combustion volume, pollution control equipment, and ash handling systems of a furnace.
5. 7. 1 Petroleum and sources
Petroleum is a naturally occurring liquid found beneath the earth’s surface that can be refined into fuel. Petroleum is a fossil fuel, meaning that it has been created by the decomposition of organic matter over millions of years. Petroleum is formed when large quantities of dead organisms–primarily zooplankton and algae–underneath sedimentary rock are subjected to intense heat and pressure. Sedimentary basins, where ancient sea beds used to lie, are key sources of petroleum. Oil is drilled all over the world. However, three primary sources of crude oil set reference points for ranking and pricing other oil supplies: Brent Crude, West Texas Intermediate, and Dubai and Oman.
5. 7. 2 Composition
Element | Percentage Range |
Carbon | 83 to 85 % |
Hydrogen | 10 to 14 % |
Nitrogen | 0. 1 to % |
Oxygen | 0. 05 to 1. 5 % |
Metals | <0. 1 % |
Sulfur | 0. 05 to 6. 0 % |
5. 7. 3 Refining
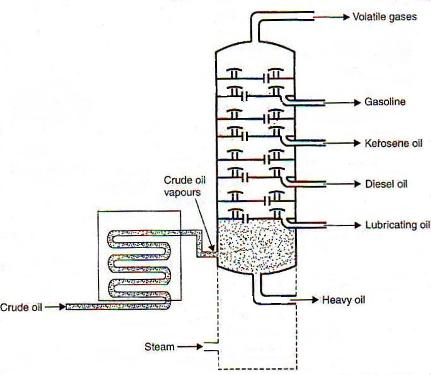
The separation of miscible liquids into their simpler forms is called fractional distillations. This separation begins when the mixture is heated at a certain temperature where the fractions of the mixture start to vaporize. Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil, and kerosene. The distillation process helps in separating these components effectively.
Crude oil is added to the chamber and is heated with high-pressure steam. The mixture starts boiling and vapor is formed. At this point, various substances enter into the vapor phase. The vapor rises in the fractional distillation column which consists of several plates. The plates have holes that allow the vapor to pass through them. The temperature is usually kept low at the top of the fractionating column. Here, components with the highest boiling point will condense in the lower part of the column while substances with a low boiling point will condense at the top. The condensed vapors or liquid fractions are then removed from the sides of the column. The collected liquid fractions can further be passed through condensers to cool them even more.
|
5. 7. 4 Octane and Cetane number
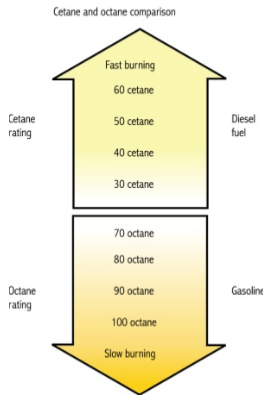
Cetane number and octane number both are used to measure the tendency of the fuel ignition. Cetane ignites very easily under compression, so it was assigned a cetane number of 100, it ignites relatively at low temperature. The Research Octane number and Motor Octane number are used in the determination of a single-cylinder test engine in the laboratory while Road Octane Number measures the antiknock performance in an actual vehicle under road driving conditions. Cetane rating indicates the cold starting ability of diesel fuel that is mostly recommended by the automakers of about 45. A high cetane rating means the fuel will ignite easily from heat and pressure and burn quickly.
|
Cetane Rating
Fuel | Research Octane Number | Motor Octane Number | Cetane Number | Boiling Point (oC) |
Ethanol | 107 | 89 | 5 | 79 |
Methane (LPG) | 120 | 120 | 0 | -161. 66 |
Diesel | -25 | - | 45-55 | 140-360 |
Gasoline | 92-98 | 80-90 | 0-5 | 37-205 |
Key Takeaways:
1) Petroleum is a naturally occurring liquid found beneath the earth’s surface that can be refined into fuel.
2) Petroleum has been created by the decomposition of organic matter over millions of years.
3) The separation of miscible liquids into their simpler forms is called fractional distillations.
4) Cetane rating indicates the cold starting ability of diesel fuel that is mostly recommended by the automakers of about 45.
5. 8. 1 Gaseous fuel
They are non-evenly distributed all over the world mainly reserve in Russia and the middle east due to the same reason as to form liquid fuels there is diversity in their prices in different regions in the world.
5. 8. 2 Natural Gas
Methane is the main constituent of natural gas and accounting for about 95% of the total volume. Other components are Ethane, Propane, Butane, Pentane, Nitrogen, Carbon Dioxide, and traces of other gases. Very small amounts of sulfur compounds are also present. Since methane is the largest component of natural gas, generally, properties of methane are used when comparing the properties of natural gas to other fuels. Natural gas is a high calorific value fuel requiring no storage facilities. It mixes with air readily and does not produce smoke or soot. It did not contain sulfur. It is lighter than air and disperses into the air easily in case of a leak.
5. 8. 3 CNG
This is the fuel that can replace gasoline, diesel fuel, and LPG. It produces fewer undesirable gases than other fuels. It is made by compressing natural gas which is mainly composed of CH4 to less than 1% of the volume it occupies at standard atmospheric pressure. CNG can be stored and distributed in hard containers at a pressure of 20 – 25 MPa mainly in cylindrical or spherical shapes.
Properties:
Chemical formula: CH4
Energy content: 9 MJ/L
Storage pressure: 20 – 25 MPa
Air: Gas combustion ratio: 10: 1
Operating Pressure: 1. 1 kPa
Density (vs Air):. 5537: 1
Cylinder weight: 1
State: Gas
Key Takeaways:
1) Methane is the main constituent of natural gas and accounting for about 95% of the total volume.
2) Natural gas is a high calorific value fuel requiring no storage facilities.
3) CNG is the fuel that can replace gasoline, diesel fuel, and LPG.
4) CNG can be stored and distributed in hard containers at a pressure of 20 – 25 MPa mainly in cylindrical or spherical shapes.
5. 9. 1 Electrochemical energies
The deterioration of materials by the chemical process is called corrosion. In electrochemical corrosion M→M+ + e- is facilitated by the presence of a suitable electron acceptor and some time it is also called a depolarizer. Corrosion can also be viewed as the spontaneous return of metals to their ores, the abandoned amount of energy used that was consumed in the mining, refining into useful objects is dissipated by a variety of different routes.
Corrosion Cells and reactions: The occurrence of oxidation and reduction steps in corrosion results in the separation of metal’s location. This can be possible due to the conductive property of metal so that electrons can flow through metal from anodic to cathodic regions. Water presence plays a major role in transportation. Fe → Fe2+ + 2e- The metal is under pressure or is isolated from the air. The metal ions dissolve in the moisture film and e- migrates to another location. The anodic process is Fe(s) → Fe2+ (aq) + 2e- While cathodic steps involve the reduction of oxygen gas O2 + 2H2O(aq) + 4e- → 4OH- Or the proton reduction H+ + e- → ½ H2(g) Or the reduction of metal ion M2+ + 2e- → M(s) Since both the cathodic and anodic steps must take place for corrosion to occur, the prevention of either one will stop corrosion. The most obvious strategy is to stop both processes by coating the object with paint or other protective coatings. Even if this is done, there are likely to be places where the coating is broken or does not penetrate, particularly if there are holes or screw threads. A more sophisticated approach is to apply a slight negative charge to the metal, thus making it more difficult for the reaction to take place: M⟶M2++2e−. |
Key Takeaways:
1) The deterioration of materials by the chemical process is called corrosion.
2) Corrosion can also be viewed as the spontaneous return of metals to their ores, the abandoned amount of energy used that was consumed in the mining, refining into useful objects is dissipated by a variety of different routes.
3) The most obvious strategy is to stop both processes by coating the object with paint or other protective coatings.
5. 10. 1 Electrolysis conductivity of electrolytes
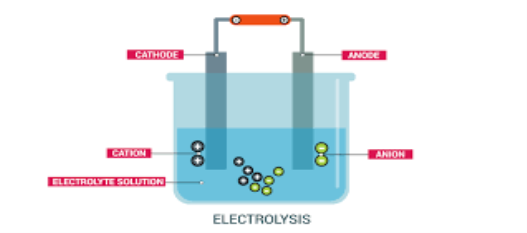
Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form. The cations are reduced at the cathode and anions are oxidized at the anode. For example, acidified or salt-containing water can be decomposed by passing electric current to their original elements hydrogen and oxygen. Molten sodium chloride can be decomposed into sodium and chlorine atoms.
|
Electrolysis is usually done in a vessel named ‘electrolytic cell’ containing two electrodes (cathode and anode) connected to a direct current source and an electrolyte which is an ionic compound undergoing decomposition, in either molten form or a dissolves state in a suitable solvent.
5. 10. 2 Factor affecting the conductivity of electrolytes
1) Nature of electrolyte: Strong electrolyte ionize completely thus conduct electricity while weak electrolyte ionizes partially and hence conduct a small extent of electricity
2) Size of ion and their salvation (mixing with solvent): Greater the size of the ion or salvation less is the conductance.
3) Nature of solvent & viscosity: Electrolyte ionizes more in a polar solvent. The greater the polarity of the solvent, the greater is the ionization, and hence greater is the conductance. Similarly, greater the viscosity of the solvent less is the conductance.
4) Concentration of the solution: Higher the concentration less is the conductance.
Key Takeaways:
1) Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form.
2) The cations are reduced at the cathode and anions are oxidized at the anode.
3) Molten sodium chloride can be decomposed into sodium and chlorine atoms.
Batteries:
The electrical interconnection of two or more electrochemical cells, each of which contains two electrodes and an electrolyte is called a Battery. The condition at which the battery is properly working is the supply of electric power, the positive terminal is cathode while the negative terminal is the anode.
Primary (Lithium cell)
It consists of lithium anode with solid electrolyte or liquid electrolyte and solid or liquid cathode. A thin protective insulating film is formed on the lithium anode protecting the anode against corrosion as it is conducive to lithium ions but not electrons while water and alcohol never form such film.
Lithium iodide solid cathode cell consists of iodine PVP cathode with 3V voltage. It is highly stable and dependable and hence used in medical sources for electronic flash guns of cameras.
Lithium-Ion Cells
Anode: Graphite, Carbon compound.
Cathode: Oxide of Lithium
Uses:
Used in Laptops, cellular phones, electronic vehicles.
Secondary Batteries:
Lead-Acid Storage Battery & Lithium-Ion Battery:
A lead storage battery is the most common device used to store energy in a portable form. This is also called a lead-acid battery. Although the batteries are reliable, which contain acidic material inside that required a proper disposal method after its complete use. These batteries have moderate power density and a good time. The battery consists of lead grids on its electrodes. The anodic grid opening is filled with spongy lead while the cathodic grid consists of lead oxide (PbO2).
Charge Chemistry of the battery:
Charge batteries are those batteries that can be recharged after a single-use. In this type of battery, each plate contains a negative as well as a positive end. The negative plate is of lead while the positive plate is made up of lead oxide in an electrolyte of approx 4. 0 M sulphuric acid.
Negative plate reaction: PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO4–(aq) Positive plate reaction: PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– Combining these two reactions, the overall reaction is the reverse of the discharge reaction: 2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq)
Discharge Chemistry of the Battery: The positive and negative plate of the batteries becomes lead sulphate. Due to the loss of sulfuric acid from electrolytes it becomes the water. Negative plate reaction: Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2e– Positive plate reaction: PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l) Combining these two reactions, one can determine the overall reaction: Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq) → 2PbSO4(s) + 2H2O(l)
|
Key Takeaways:
1) The electrical interconnection of two or more electrochemical cells, each of which contains two electrodes and an electrolyte is called a Battery.
2) It consists of lithium anode with solid electrolyte or liquid electrolyte and solid or liquid cathode.
3) A lead storage battery is the most common device used to store energy in a portable form.
4) Charge batteries are those batteries that can be recharged after a single-use.
5. 12. 1 Lithium-Ion Battery
Lithium metals are used as a battery anode material because of their lightweight, low electrode potential, high electrochemical equivalence, and good conductivity. These batteries are a type of rechargeable battery. These types of batteries are commonly used for portable electronics and electric vehicles. Lithium batteries are classified into primary and secondary batteries. A primary battery is not chargeable and the secondary battery is chargeable. Based on cathode material used lithium primary cells are classified as:
- Soluble-cathode cells: In this type of cells liquid or gaseous cathode material, such as sulphur dioxide or thionyl chloride.
- Solid-cathode cells: These materials used solid material for cathode substances such as V2O5, MnO2, CuO, etc.
- Solid electrolyte cell: This type of cell use electrolytes in the solid form itself as the cathode. E. g. - PbI2, PbS etc. Are used as solid electrolyte cathodes.
Advantages of Lithium batteries:
• High cell voltage, up to above 4 V, depending on the cathode material. This is because of the very negative electrode potential of Li/ Li+.
• High energy density due to the low atomic mass of lithium. 1F is released by the dissolution of 7 g of the metal.
• Operation over a wide temperature range, from about 70 to – 40°C.
• Flat discharge characteristics-constant voltage and resistance through most of the discharges of many lithium cells.
5. 12. 2 Nickel Cadmium battery
Nickel-Cadmium: Nickel-cadmium battery is a type of rechargeable battery that uses nickel oxide hydroxide and metallic cadmium as electrodes. The maximum recharge rate for the Ni-Cd battery varies by size.
Voltage: Zinc carbon primary cells and Nickel-cadmium cells are not preferably exchangeable because the potential of Zinc-Carbon primary cells is greater than that of Ni-Cd cells. Many electronic devices are designed to work with a primary cell that may discharge as low as 0. 90 to 1. 0V per cell.
Charging: Depending on the cell manufacturing Ni-Cd batteries can be charged in different ways. The charge rate is measured based on the percentage of the amp-hour capacity the battery is fed as the steady current over the duration of charge.
On fully charging the Ni-Cd batteries the batteries consist of a nickel oxide hydroxide positive electrode plate, a cadmium negative electrode plate, a separator, and an alkaline electrolyte. Nickel Cadmium consists of nickel wire gauze and electrode grids. The anode grid consists of a mixture of spongy cadmium with 78% cadmium hydroxide, 18% iron, 1% nickel, and 1% graphite. The cathode grid contains nickel hydroxide (80%), cobalt hydroxide (2%), graphite (18%), and traces of barium compound. Graphite increases the conductivity, the cobalt and barium compounds increases the efficiency of active material and also the cycle life. 6M KOH is the electrolyte.
Electrode Reaction: On anode, Cd + 2OH- Cd(OH)2 + 2e- On cathode end, 2Ni(OH)3 + 2e- 2Ni(OH)2 + 2OH- Cd + 2Ni(OH)3 2Ni(OH)2 + Cd(OH)2 Applications: These are used in pocket calculators, photo flash units, cordless garden tools, alarm, emergency lighting, instrument etc.
|
5. 12. 3 Fuel cells
A fuel cell is an electrochemical cell that helps in the conversion of chemical energy of a fuel and oxidizing agent into electricity with the help of a redox reaction. The fuel cell has to be supplied by an external source of fuel and an oxidant. The hydrogen or any other fuel is oxidized electrochemically inside the fuel cell to produce a potential difference i. e. a voltage capable of producing a working currently. The overall chemical reaction in a hydrogen fuel electrochemical cell involves the oxidation of hydrogen by oxygen to produce only water. Hydrogen fuel cells offer an alternative to rechargeable cells and batteries. A fuel cell will produce a potential difference and a workable electric current until one of the reactants is used up.
Hydrogen gas can be used as fuel:
2H2(g) + O2(g) 2H2O(l)
It is an exothermic reaction, releasing lots of heat energy when burnt, remember the 'squeaky pop' lit splint test for hydrogen. The hydrogen-oxygen fuel cell is non-polluting since only water is produced.
|
A hydrogen-oxygen fuel cell is a non–polluting clean fuel. Fuel cells do not produce pollutants like carbon monoxide, sulfur dioxide, and nitrogen oxides. Cars powered by fuel cells would be quite an environmental advantage in cities, where electric cars are already beginning to be significantly used in developed countries. Fuel cells could replace larger batteries that are not easily recycled and contain highly toxic metal compounds. It would be an ideal fuel on this basis e. g. for motor vehicles, but that's not the only factor to consider. It would be ideal if the hydrogen fuel could be manufactured by electrolysis of water e. g. using solar cells.
Key takeaways:
1) Lithium batteries are a type of rechargeable battery.
2) A primary battery is not chargeable and the secondary battery is chargeable.
3) A fuel cell is an electrochemical cell that helps in the conversion of chemical energy of a fuel and oxidizing agent into electricity with the help of a redox reaction.
4) Fuel cells could replace larger batteries that are not easily recycled and contain highly toxic metal compounds.
REFERENCE BOOKS:
- A textbook of engineering chemistry by M. M. Uppal
- Applied Chemistry by Krishnamurthy P. Vallinayagam and K. Jeysubramanian TMH Publication
- A textbook of engineering chemistry by Shashi Chawla
- A textbook on experiment and calculations in engineering chemistry by S. S. Dara, S. Chand Publication
- Engineering Chemistry by R. V. Gadag and A. N. Shetty
- Textbook of polymers science by F. W. Billmer, John Wiley and sons
- University chemistry, Mahan, Pearson education