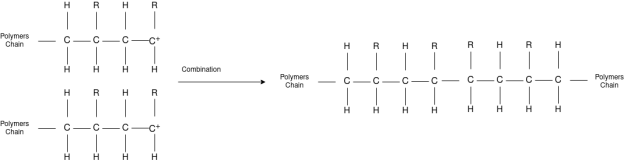
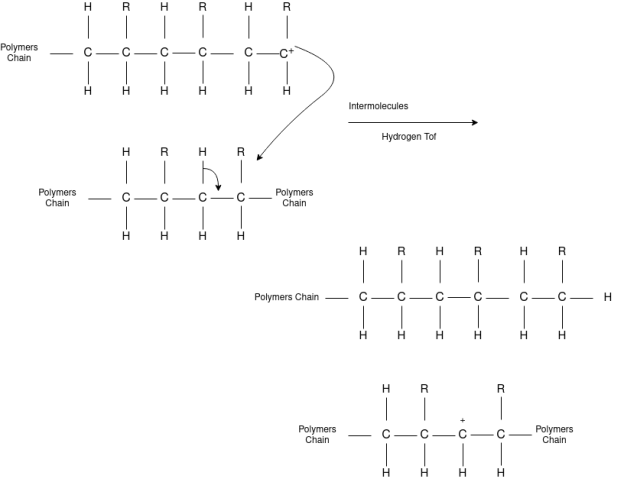
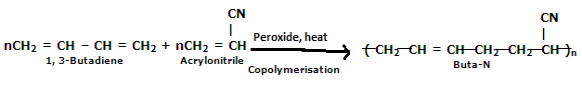Polymer chemist study large, complex molecules that are built up from many smaller units. They study about the smaller building blocks combine, and create useful materials with specific characteristics by manipulating the molecular structure of the monomers/polymers used, the composition of the monomer/polymer combinations, and applying chemical and processing techniques that can to a large extent, affect the properties of the final product. Polymer chemists are unique within the chemistry community because their understanding of the relationship between structure and property spans from the molecular scale to the macroscopic scale.
It is a long molecule formed by joining together of thousands of small molecular units by chemical bonds. Polymers are giant molecules also called macromolecules that are essential to our existence. They are important chemicals in our bodies in plants (starch, cellulose) and in our everyday lives (fibers, plastics, elastomers). Polymers are made by transforming small molecules into molecules with very large molecular weights. Although the chemical properties of polymers are similar to those of analogous small molecules, their physical properties are quite different. Every polymer has its own characteristics, but most polymers have the following general properties:
- Polymers can be very resistant to chemicals.
- Polymers can be both thermal and electrical insulators.
- Generally, polymers are light in weight with varying degrees of strength.
- Polymers can be processed in various ways to produce thin fibers or intricate parts.
- The polymer is a molecule formed by the joining of thousands of smaller molecular units together by chemical bonds. A chemical process that leads to the formation of a polymer is known as polymerization.
Functionality: The number of bonding sites or reactive sites or functional groups present in the molecule. Ex: The double bond in vinyl monomers (CH2 = CHX) can be considered as a site for two free valencies. When the double bond is broken, two single bonds become available for combination.
H2C=CHX → CH2 – CHX
- When the functionality of monomer is two bi-functional linear straight-chain polymers is formed.
Ex: (a)vinyl monomers
(b) adipic acid
(c) hexamethylene diamine
(d) terephthalic acid
(e) ethylene glycol
(f)amino acid Example for polymer: HDPE (high-density polythene)
When the functionality of the monomer is three (tri-functional), a three-dimensional network polymer is formed. Ex: phenol, glycerol Examples for polymers: Urea-formaldehyde, phenol-formaldehyde.
Key Takeaways:
1) It is a long molecule formed by joining together of thousands of small molecular units by chemical bonds.
2) The polymer is a molecule formed by the joining of thousands of smaller molecular units together by chemical bonds.
3) A chemical process that leads to the formation of a polymer is known as polymerization.
|
Monomers: Monomers are atoms that bond together to form more complex structures such as polymers. There are four main types of monomer i.e.; sugars, amino acids, fatty acids, and nucleotides. Each of these monomer types plays important role in the existence and development of life and each one can be synthesized abiotically. Monomers are commonly found in the interstellar medium, nebulae, and chondritic meteorites. The number of repeating units (n) in the chain so formed is called the Degree of polymerization (DP=n). Polymers with a high degree of polymerization are called High polymers and those with a low degree of polymerization are called Oligo polymers
Functionality: The functionality of a monomer determines the final polymer that will be formed due to the combination of the monomers. The number of reactive bonds that are available for coupling will determine whether the monomer will be mono-, bi-, tri-.
Mono-functional: The presence of a single reactive group in a monomer molecule, then it is termed as mono-functional. However, a mono-functional group cannot lead to the propagation of a polymer chain. E.g.: in carboxylic acid, CH3COOH, the –COOH group is the mono-functional group.
Bi-functional: The presence of two reactive groups in the monomer molecule is termed as bi-functional. Polymerization reaction with bi-functional groups occurs when a double bond splits to couple with another double-bonded monomer. If a double-bonded molecule is present, then the polymer would be –
nR=R -(R-R)n-
Key Takeaways:
1) The atoms that bond together to form more complex structures such as polymers are monomers.
2) Monomers are commonly found in the interstellar medium, nebulae, and chondritic meteorites.
3) The presence of a single reactive group is mono – functional.
4) The presence of two reactive groups in the monomer molecule is termed as bi-functional.
Addition polymerization:
The addition polymerization is the process in which the linking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerization and the polymer is an exact multiple of the original monomeric molecule.
Condensation polymerization:
An intermolecular reaction involving two different functional reactants with an affinity for each other and taking place through repeated condensation reaction is known as condensation polymerization.
Key Takeaways:
1) The linking together of monomer molecules by a chain reaction.
2) No molecule is evolved during polymerization.
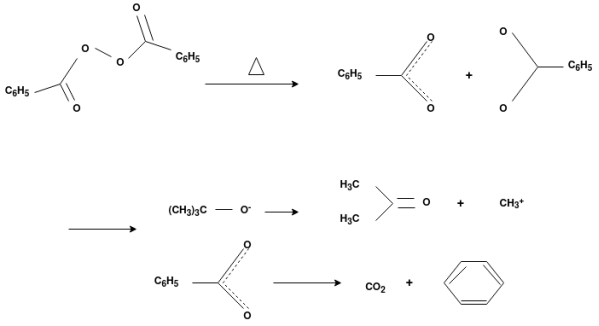
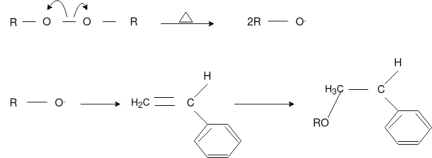
Free Radical Polymerization:
The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called free radical polymerization. In order to obtain a wide variety of different polymers and material composites, free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction makes it one of the most versatile forms of polymerization which allow a facile reaction of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.
|
Radical Initiators
|
Chain Initiation
|
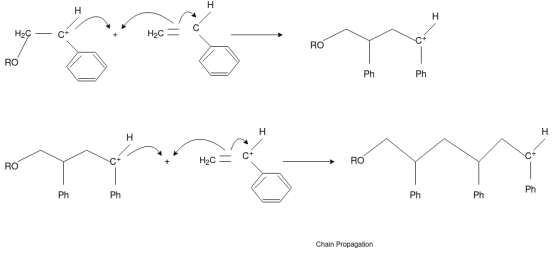
Chain Propagation
|
Chain Termination
|
Chain Transfer Reaction
Key Takeaways:
1) The successive addition of free-radical building blocks through the polymerization is called free radical polymerization.
2) To obtain a wide variety of different polymers and material composites, free radical polymerization plays a major role in it.
3) The most common and thermodynamically favored chemical transformations of alkenes are addition reactions.
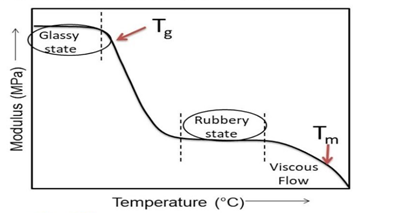
The glass transition temperature is described as the temperature at which 30–50 carbon chains start to move. At the glass transition temperature, the amorphous regions experience the transition from a rigid state to a more flexible state making the temperature at the border of the solid-state to a rubbery state. It is also said that at this temperature the free volume (the gap between the molecular chains) increases by 2.5 times. Glass transition temperature is represented by Tg and is a property of the amorphous materials or the amorphous portion of semi crystalline materials. When the ambient temperature is below Tg, the molecular chains of amorphous materials are frozen in place and behave like solid glass. Plastic materials with flexible backbone show lower Tg, whereas plastic materials whose molecular structure is stiff, rigid, and inflexible show a higher Tg. Glass transition temperature helps determine various flexible and rigid applications for a material.
|
Polymers are hard and brittle like glass, due to the lack of mobility below the Glassy state (Tg)
Polymers are soft and flexible like rubber due to some mobility above Glassy State (Tg)
The physical and polymer properties of the polymer also change above the Glassy State (Tg)
The melting point also known as melt temperature is the critical temperature above which the crystalline regions in a semi-crystalline plastic are able to flow. Semi-crystalline polymers begin to soften above Tg, however, they do not demonstrate fluid behavior until the Tm range is achieved. In general, Tm for a semi-crystalline polymer is higher than its Tg. At temperatures above Tg but below Tm, there is a “rubbery region,” where the material can exhibit large elongations under relatively low load. Plastic materials are made up of uneven chain lengths and require a different amount of energy to move which means that Tg or Tm for amorphous and semi-crystalline polymers is not one definite temperature but a range of temperature during which all the chains start to move and experience complete flow. At temperatures above the melting point, semi-crystalline plastics exhibit a rapid phase change from solids to viscous liquids that can be moulded or given shapes. Even though, amorphous material starts transitioning into a leathery region at Tg and continues to soften over a wide range of temperatures.
Key Takeaways:
1) The amorphous regions experience the transition from a rigid state to a more flexible state making the temperature at the border of the solid-state to a rubbery state.
2) Plastic materials with flexible backbone show lower Tg, whereas plastic materials whose molecular structure is stiff, rigid, and inflexible show a higher Tg.
3) At temperatures above Tg but below Tm, there is a “rubbery region,” where the material can exhibit large elongations under relatively low load.
Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect their cost.
Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behavior. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called cross-links, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, there most, as opposed to a thermoplastic, cannot return to their initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression moulding, resin transfer moulding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behavior. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
Properties:
Property | Thermoplastics Polymer | Thermosetting Polymer |
Molecular Structure | Linear polymer: weak molecular bonds in a straight-chain formation | Network polymers: high level of crosslinking with strong chemical molecular bonds |
Melting point | Melting point lower than the degradation temperature | Melting point higher than the degradation temperature |
Mechanical | Flexible and elastic. High resistance to impact (10x more than thermosets). Strength comes from crystallinity | Inelastic and brittle. Strong and rigid. Strength comes from crosslinking. |
Polymerization | Addition polymerization: repolymerized during manufacture (before processing) | Polycondensation polymerization: polymerized during processing |
Microstructure | Comprised of hard crystalline and elastic amorphous regions in its solid-state | Comprised of thermosetting resin and reinforcing fibre in its solid-state |
Size | Size is expressed by molecular weight | Size is expressed by crosslink density |
Solubility | Can dissolve in organic solvents | Do not dissolve in organic solvents |
Service temperature | Lower continuous use temperature (CUT) than thermosets | Higher CUT than thermoplastics |
Key Takeaways:
1) The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements.
2) A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating.
3) The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape.
4) A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition.
Compounding is the best method for changing the characteristic of engineered thermoplastics. The final compound product is a blend of plastics and additives. Plastic compounding companies in NY can help you create beautiful and strong products. With the help of melt blending plastics, it is possible to change characteristics, such as:
Physical
Electrical
Thermal
Aesthetic
All compounding begins with polymers or base resins. Different resin systems exist, and so you must choose the proper one with the correct characteristics for your final product. Once a base resin is picked, it is time to determine the specific additives, reinforce or fillers that will be incorporated into the compounded plastic. This process allows the final product to be pre-colored or flame retardant. It can also make plastic more or less conductive, while also strengthening final parts. There are several steps taken when creating specialty compounding. In the end, pellets are sent to customers for sheet extrusion or injection moulding.
Compounding consists of preparing plastic formulations by mixing and/or blending polymers and additives in a molten state to achieve the desired characteristics. These blends are automatically dosed with fixed setpoints usually through feeders/hoppers. It is mostly a blend of copolymers such as ABS, SAN, SMA, etc. with additives such as anti-oxidants, UV-stabilizers, and other value-adding agents, and sometimes a strengthening component is added such as glass fiber.
Key Takeaways:
1) Compounding is the best method for changing the characteristic of engineered thermoplastics.
2) Compounding consists of preparing plastic formulations by mixing and/or blending polymers and additives in a molten state to achieve the desired characteristics.
Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behavior. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called cross-links, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, there most, as opposed to a thermoplastic, cannot return to their initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression moulding, resin transfer moulding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behavior. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
Key Takeaways:
1) A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating.
2) The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape.
3) A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition.
Steps involved in the Injection Moulding:
The various stages of the injection moulding process are carefully considered when analyzing part design, tool creation, and efficient production of moulded plastic products. There are a lot of factors and configurations which we won’t touch on here but the basic process is the same. Let’s start with the basics.
(The heated plastic is injected into the mould. As the melt enters the mould, the displaced air escapes through vents in the injection pins and along the parting line. Runner, gate, and vent design are important to ensure the mould is properly filled.)
(Once the mould is filled the part is allowed to cool for the exact amount of time needed to harden the material. Cooling time is dependent on the type of resin used and the thickness of the part. Each mould is designed with internal cooling or heating lines where water is cycled through the mould to maintain a constant temperature)
STEP 4: PLASTICIZING THE RESIN
(While the part cools, the barrel screw retracts and draws new plastic resin into the barrel from the material hopper. The heater bands maintain the needed barrel temperature for the type of resin being used.)
(The mould opens and the ejector rod moves the ejector pins forward.
The part falls and is captured in a bin located below the mould.)
STEP 6: REMOVING THE RUNNER AND PACKAGING
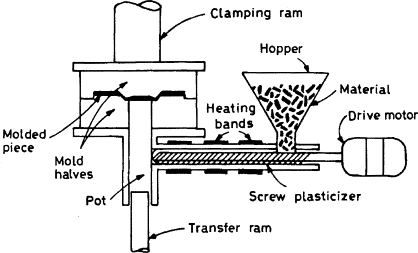
Transfer Moulding: Transfer moulding is similar to compression moulding; however, the material is first placed in a transfer chamber before entering the mould. As in compression moulding, thermosets that are cross-linked with heat are used for transfer-moulding applications. Multiple cavities can be used within transfer moulding since the material is entering the mould after the mould is closed. Since runners and sprues are present, shear is created. This facilitates heat needed for cross-linking and flow to the cavities. Transfer-moulding machines are also generally positioned with the moulds opening vertically.
Since the mould is closed and clamped before the material entering the mould, there is no presence of flash with transfer moulded parts. The dimensions of the final parts are very accurate due to the flow of the polymer being gated. Another advantage is that the cure time is faster since there is the presence of shear flow, which creates heat. Inserts can also be used to create more complex parts than can be created by compression moulding.
|
Shorter production cycles for higher weight parts
Transfer molding offers shorter production cycle times than traditional molding techniques, such as compression molding, as the compound preparation and product finishing time are greatly reduced. This means there are less cutting and flash. Cure times are reduced since the rubber enters the mold cavity at a higher and even temperature, and can thus begin to cure more quickly.
Tighter dimensional tolerance. The overall process also allows for much tighter tolerances, leading to more complex parts, which is difficult to achieve with compression molding. Given that the mold is not held open by surplus material spilling out of the cavity parting line, any excess holds the plunger open from the pot, therefore not affecting the actual part being produced.
Provides better uniformity. The pot and plunger design allow for more standardization and lower costs of tooling, and the process is more consistent than Compression molding, with fewer variables. The fact that the mold is closed before accepting the shot means that the parts are more dimensionally consistent across a production run.
Reduced tooling lead times compared to a full Injection tool. In relation to Injection Molding, Transfer Molding tooling offers much shorter lead-times with fewer ancillary features required. This means that the production run is ready faster with far less tooling costs.
More accurate and consistent than compression molding Transfer molding allows for much sharper edges since rubber enters the cavity near the curing temperature. This vastly reduces any tendency for “backrinding”, which can be a risk with Compression molding. This occurs when rubber cures unevenly in a mold, and the action of expansion, cure and shrink tears off pieces of rubber around the split line of a mold. This is countered by increasing the width of the split line in a Compression tool, but greatly increases the overall deflating necessary as a consequence. Transfer Molding generally eliminates this issue, and thus allows for sharper split lines.
More expensive tooling than a Compression mold. Due to the more complex nature of the design of the molds, tooling investment can be somewhat higher.
Slower production cycle than an injection tool. The Transfer molding process is usually slower than an injection tool, sometimes limiting the overall production rate, as changeover times can be somewhat extended.
Manual handling of the piston can be a problem
The skill level is often proportionately higher and for larger parts or tools, manual handling can become somewhat of an issue.
Extrusion moulding: Extrusion moulding (also known as plasticization extruding) is a process that the stack of powders or the green body in die is pushed out to assume another form of green body or other final product under pressure. The cold extruding process is applied to mixtures of metal powders and organic binders, and extruding is performed at low temperatures (40–200 °C) to form the green body. The processes include material preparation, preprocessing, extruding, cutting, and reforming. The porous products can be obtained after drying, pre-sintering, and sintering of the extruded green body. It is an effective way to produce a long porous tube with a small diameter. The pretreatment of a mixture under pressure involves making full contact between the plasticizer and particle surfaces, removing the gas inclusion, and finally to ensure uniform density. Plasticizers have a large effect on a material’s properties. Therefore, certain requirements are needed for them, including that there should not be any reaction with porous materials during sintering, and that they should be removable, sticky, and have the great pore-forming ability. The plasticizers in common use are olefin, amylum, and polythene alcohol. Powders will be subjected to pressure from the sidewall, friction from either the powder and the wall, or the extrusion shaft and the wall, in addition to the normal compression from the extrusion shaft. The key factors affecting the properties of green body extrusion are the types of powders, the particle shape and size, the plasticizer type and content, the precision of the mold, the pressure from extrusion, the extrusion speed, and the preheating temperatures. The selection of the preheating temperature depends on the optimal plastic used for the plasticizer at the selected temperature. The extrusion speed can be determined experimentally, and it is closely related to the particle size, shape, extrusion ratio, fluidity, extrusion force, and plasticizer. Higher extrusion speeds may cause the green body to crack.
Key Takeaways:
1) Extrusion molding is a process that the stack of powders or the green body in die is pushed out to assume another form of green body or other final product under pressure.
2) The selection of the preheating temperature depends on the optimal plastic used for the plasticizer at the selected temperature.
Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers. The most common vulcanization agent is sulfur. It forms bridges between individual polymer molecules when heated with rubber. Often a catalyst and initiator are added to accelerate the vulcanization process. The cross-linked elastomers have many improved mechanical properties. In fact, un-vulcanized rubber has poor mechanical properties and is not very durable.
- Mixing of crude rubber with about 5-30% of sulfur (cross-linking agent) and other additives such as:
- activator (commonly zinc oxide or stearic acid),
- accelerator (guanidines, thiazoles, dithiocarbamates, xanthates, thiurams),
- coagulants (acetic acid, calcium chloride),
- anti-oxidants (amines, phenolics, phosphites),
- color pigments,
- surfactants,
- softeners,
- ant-foaming agents,
- anti-tack agents (Rosin derivates, coumarone-indene resins, aliphatic petroleum resins, alkyl-modified phenol-formaldehyde resins).
Slow cross-linking starts at this stage. It is necessary to avoid active vulcanization during mixing, which may cause cracks formation at the moulding stage.
- Moulding (shaping) the rubber mixture. The rubber must be shaped before the heating stage since cross-linking makes shaping impossible.
- Heating the mixture to 250-400ºF (120-200ºC). The increased temperature speeds up the vulcanization process resulting in fast and complete cross-linking. C-S bonds replace C-H bonds linking chain poly-isoprene molecules. Each link is formed by one to seven sulfur atoms.
Applications:
(i) The major application of natural rubber is in the manufacture of tyres.
(ii) In heavy-duty tyres, the major portion of the rubber used is natural rubber.
(iii) The tank linings in chemical plants where corrosive chemicals are stored are prepared from rubber.
(iv) To reduce machine vibrations, rubber is used for sandwiching between two metal surfaces.
(v) Foam rubber is used for making cushions’, matrices, padding, etc. toys and sports items are manufactured from natural rubber.
Key Takeaways:
1) Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers.
2) The cross-linked elastomers have many improved mechanical properties.
3) Un-vulcanized rubber has poor mechanical properties and is not very durable.
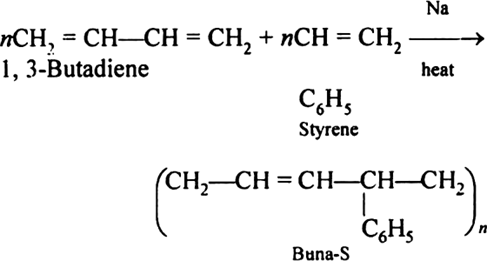
Preparation of Buna-S:
Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrene. Buna-S is the replacement of natural rubber while styrene, 2 monomers, and butadiene play a major role in its derivation whereas these 2 monomers are polymerized by two different processes i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called cold rubber. The obtained rubber is called Styrene-Butadiene Rubber (SBR).
|
Applications:
(i) Buna-S is used as the natural rubber i.e., they are widely used in pneumatic tyres.
(ii) SBR is extensively used in coated papers.
(iii) They are highly used in building materials as a binding material.
(iv) SBR is also used as a binder in lithium-ion battery electrodes.
Preparation of Buna-N:
Buna-N is commonly used as elastomers. They are unsaturated synthetic copolymers. They are obtained by the copolymerization of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst. Nitrile rubber, NBR, or Perbunan are other terms used for the Buna-N. They are synthetic rubber copolymer of acrylonitrile (ACN) and butadiene. Elastomers are natural or synthetic material that does not break when stretched and when released it returns to its original length. The most important monomer used in preparing Buna-N is Acrylonitrile rubber which gives hardness, tensile strength, fuel, and oil resistance. It usually contains 34% ACN. Grinding wheels can be made with nitrile rubber. When they are used for grinding smokes or fumes are emitted, which are known as acrid. The objectionable odor can be prevented if certain classes of diketones, which contain a conjugated system of two double bonds or unsaturated groups are added. Dibenzoylethylene, chloranil, and anthraquinone help to prevent odor formation.
|
Buna-N is resistant to oil, fuel, and other chemicals which means more Buna-N higher the resistance to oil but the flexibility of the material is less. They are resistant to aliphatic hydrocarbons. They are non-resistant to solvents but they may be attacked by ozone, ketones, esters, and aldehydes.
Applications:
(i) They are used in disposable non-latex rubbers, belts, gaskets.
(ii) They are used in the preparation of adhesive and as a pigment binder.
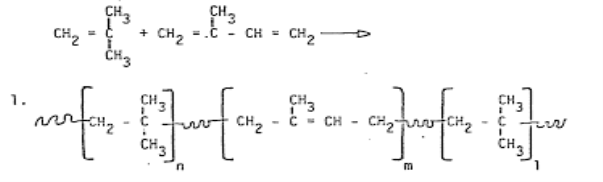
Preparation of Butyl rubber:
|
Applications:
- Low permeability to air, gases, and moisture
- Vibration damping
- The low glass transition temperature
- Low modulus elastomer
- Low compression set Resistance to aging and to weathering from atmospheric exposure
- Wide vulcanization versatility
- Fast cure rates
- Processing safety: no nitrosamines or nitrosamines precursors
- A broad range of durometer and tensile strength properties
- Low filler content for specific gravity, cost-effective compounding
Polymers in medicine and surgery:
A polymer biomaterial when comes in contact with blood and tissues should not cause any harm or destroy components of blood or tissues. It should not be toxic, allergic. It should be easy to fabricate, sterilize without alteration in properties, in short, and biocompatible.
Applications:
- To construct an artificial replacement for human organs.
- To repair, sustain and augment functions of organ
- To provide biochemical functions.
REFERENCE BOOKS:
1) A textbook of engineering chemistry by M.M. Uppal
2) Applied Chemistry by Krishnamurthy P. Vallinayagam and K. Jeysubramanian TMH Publication
3) A textbook of engineering chemistry by Shashi Chawla
4) A textbook on experiment and calculations in engineering chemistry by S.S. Dara, S. Chand Publication
5) Engineering Chemistry by R.V. Gadag and A.N. Shetty
6) Textbook of polymers science by F.W. Billmer, John Wiley and sons
7) University chemistry, Mahan, Pearson education