Unit-2
Abrasives and Adhesives
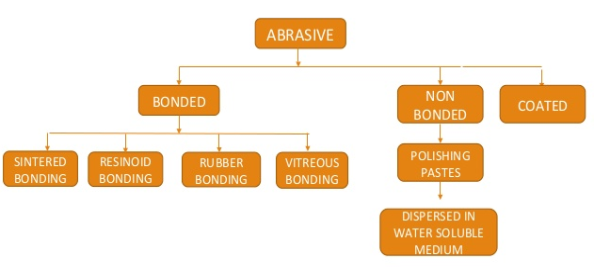
Abrasive is a sharp and hard material that is used to wear away the surface of softer, less resistant materials. Included within the term are both natural and synthetic substances, ranging from the relatively soft particles used in household cleansers and jeweler’s polish to the hardest known material, the diamond.
Abrasives are used in the form of grinding wheels, sandpapers, honing stones, polishes, cutoff wheels, tumbling and vibratory mass-finishing media, sandblasting, pulp stones, ball mills, and still other tools and products. Only through the use of abrasives is the industry able to produce the highly precise components and ultra-smooth surfaces required in the manufacture of automobiles, airplanes and space vehicles, mechanical and electrical appliances, and machine tools.
Key Takeaways:
1) Abrasive is a sharp and hard material that is used to wear away the surface of softer, less resistant materials.
2) Abrasives are used in the form of grinding wheels, sandpapers, honing stones etc.
3) The use of abrasives is the industry able to produce the highly precise components and ultra-smooth surfaces required in the manufacture of automobiles.
Natural Abrasives:
Corundum (Al2O3) [Alundum]
Properties:
- Crystalline
- Very Hard
- Moh’sscale-9
- brown to grey in color
Uses:
- In grinding wheels
- To grind glass/lens/metals,
- Ruby lasers
Diamond (C)
- Diamond exists in three major forms
- Diamond (gem-grade)
- Borts– These are diamonds that are off color or faulty
- Carbonado – These are black diamonds mined from Brazil. They have good hardness, but due to lackluster, do not find application as jewelry.
- They are commonly used as abrasives
Properties
- crystalline
- Chemically inactive
- Moh’s scale-10
Uses
- In bits of drilling points
- Sawteeth for cutting rocks
- In grinding wheels
- In engraving tools
Artificial abrasives
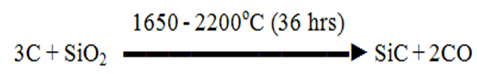
Silicon Carbide / Carborundum / Crystolon (SiC)
Preparation:
Raw materials are
- Petroleum Coke (source of carbon)
- Sand (source of Si)
- Saw Dust ( To provide hardness)
All raw materials are sized, dried, and mixed along with old charge and fed into the Acheson furnace with a little amount of NaCl (flux)
|
Properties
Melting Point is 2700oC
Mohs scale hardness is 9.3
Chemically Inert
High Thermal Stability
Brittle hence strength is less
USES
Cutting tools
Grinding of cast iron, brass, bronze, porcelain marble
Polishing leather, lenses (Abrasive paper and Cloth)
Refractory in furnace
Concrete is the most widely used non-metallic material in the construction of buildings, dams, bridges, etc. Cement act as a binder substance that is used to harden and help in binding the material together. It is used for binding material like bricks, stones, tile and also used for flooring and plastering purposes. Cement is used for construction work by mixing up sand and crushed stones. It is a mixture of sand and water which is used in binding bricks together and for plastering purpose. Based on chemical composition cement can be classified into 4 different parts:
(i) Natural Cement
(ii) Puzzolana Cement
(iii) Slag Cement
Key Takeaways:
1) Cement act as a binder substance that is used to harden and help in binding the material together.
2) It is a mixture of sand and water which is used in binding bricks together and for plastering purpose.
|
The raw material which is used for manufacturing cement contains the following materials:
- Calcareous (chalk consist of limestone)
- Argillaceous (clay consist of silicates of alumina)
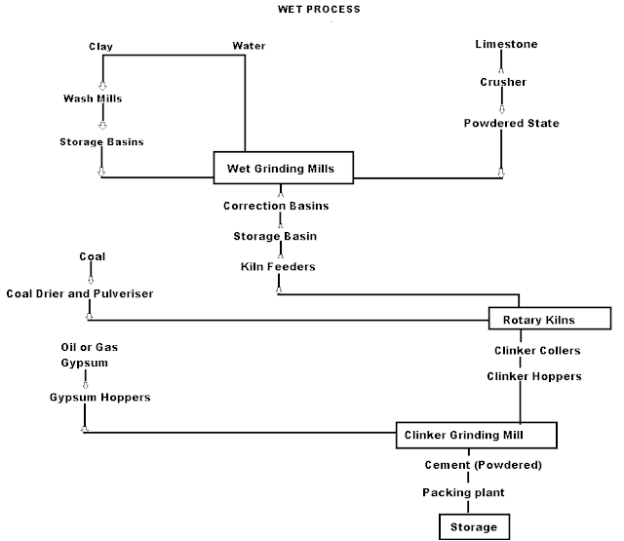
Wet Process manufacturing: On using the calcareous as raw material then chalk must be crushed into minute particles and hence dispersed in water in a wash mill. Wash mill used to break the solid materials into lumps/slurry. In similar wash mill clay is also broken up and mixed with water. Then this slurry is stored in the silo called slurry silo where it is consistently stirred. The composition of raw material is checked again and if required corrected by adding clay or chalk materials as per requirement.
|
Cement Manufacturing Process Flow Chart
(i) Drying Zones: The raw material in slurry form is directly fed into the kiln which has more amount of water. It is the upper portion of the kiln. In this zone, water is evaporated at temperatures 100-400°C.
(ii) Formations of modules: As the slurry gradually descends in the kiln, the carbon dioxide from the slurry evaporates and small lumps formed which may be called modules.
(iii) Burning Zone:- The modules enter this zone where temperatures are kept about 1400-1500° C. The modules are converted into dark greenish balls and the product obtained in the kiln, known as clinker, is of varying size 5 to 20 mm. The clinkers are very hot when coming out of this zone.
(iv) Cooling of Clinkers:- It is used to cool down the clinkers up to about 90°C.
Grinding: The cooled clinkers are finally ground in ball mills or tube mills.
Also, the gypsum is added during grinding about 2-4%. The gypsum acts as a retarder and so allows the cement to mix with sand or aggregate and to be placed in position. i.e.; it increases the initial setting time of cement.
Storage and Packing: As cement comes out from grinding mills, it is collected in a hopper and taken in a bucket elevator for storage in silos.
The cement from silos is packed by machines in bags. Each bag of cement contains 50 kg or 0.035 m3 of cement.
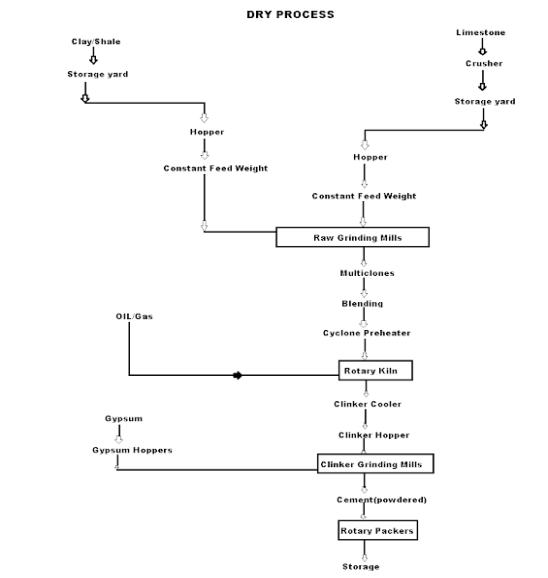
Dry Process manufacturing: This type of process for manufacturing cement is available in the places where hard crystalline limestone and shales are available. The advantage of using this type of process is that fuel consumption is low.
|
Mixing of Raw Materials: The used raw material undergoes the following process:
- Crushing: The raw materials are broken in crushers to small fragments.
- Drying: The crushed materials are dried by heating at a sufficiently high temperature. It may be done in drying kilns.
- Reduction of size: The drying materials are then ground by using ball mills and tube mills to reduce the size of materials to find powder.
- Mixing in correct proportion: The finely dried materials are mixed in exact proportions. The mixing may be done either mechanically or by pneumatic methods.
Burning and Grinding: In this process, the raw materials are mixed, fined, and then fed into the kiln whereas, in the wet process, the raw materials are crushed separately and then directly mixed in correct proportion in the presence of water to make a fine thin paste known as Slurry.
Key Takeaways:
1) This slurry is stored in the silo called slurry silo where it is consistently stirred.
2) The cooled clinkers are finally ground in ball mills or tube mills.
3) The composition of raw material is checked again and if required corrected by adding clay or chalk materials as per requirement.
Portland cement is used as the basic ingredient of concrete worldwide. It is the most common type of cement in general use. It is a mixture of calcium silicates and calcium aluminates with a small amount of gypsum in it. On mixing the powder of Portland cement with water it gives a hard, stone-like mass which resembles Portland rock this is the reason why it is named the Portland cement.
Composition of Portland Cement: - Hydraulic cement produced by pulverizing clinkers that consist of calcium silicate, usually containing one or more of the forms of calcium sulfate as anointer ground station. A good sample of Portland cement consists of:
(i) Silica(SiO2) : - 20-24%
(ii) Calcium Oxide : - 60-70%
(iii) Alumina (Al2O3) : - 5-7%
(iv) Magnesia(MgO) : - 2-3%
(v) Ferric Oxide (Fe2O3) : - 1-2.5%
(vi) Sulphur trioxide (SO3) : - 1-1.5%
(vii) Sulphur Oxide : - 1%
(viii) Potassium Oxide (K2O) : - 1%
Key Takeaways:
1) Portland cement is used as the basic ingredient of concrete worldwide.
2) It is a mixture of calcium silicates and calcium aluminates with a small amount of gypsum in it.
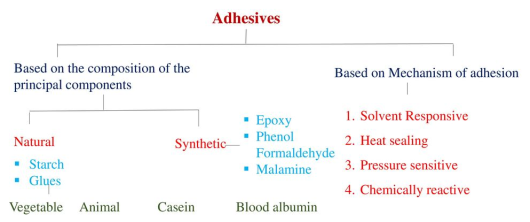
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
An adhesive is a substance that sticks to the surface of an object such that two surfaces become bonded. A typical home improvement store carries many different adhesives for many different applications. The interaction of molecules is known as intermolecular bonding or secondary bonding. Primary bonding, also known as intra-molecular bonding, is the interaction of atoms within a molecule and includes covalent and polar covalent bonding. Secondary bonding includes dipole-dipole bonding (the interaction of molecules that have a permanent net dipole moment) and hydrogen bonding (an interaction that occurs when a hydrogen atom is bonded to an N, O, or F atom in a molecule).
Key Takeaways:
1) An adhesive is a substance that sticks to the surface of an object such that two surfaces become bonded.
2) The interaction of molecules is known as intermolecular bonding or secondary bonding.
3) Primary bonding, also known as intra-molecular bonding, is the interaction of atoms within a molecule and includes covalent and polar covalent bonding.
Adhesive bonding results from physical and chemical interaction between the adhesive and substrate. The adhesive and adhered must be in intimate contact and have appropriate chemistries for this interaction to occur.
An intermediate adhesive layer is used to bond two surfaces. They are used in many industries like cars, airplanes, space shuttles.
Process:
Polymer adhesive applied to one or both surfaces.
Pressure applied to force the surface into close contact
Adhesive cured form liquid or viscoelastic state into solid-state.
Polymers are large molecule consisting of linked molecules (monomers)
Hardening
-solvent evaporation
-solidification upon cooling
-polymerization by chemical reactions
Deposition of wafer surfaces
-Spin coating, spray coating, electrodeposition, stamping, etc.
-CVD(thin films), lamination of films or sheets
|
Physical factors:
1) Surface tension
For better bond strength interfacial tension between the adhesives molecules and surface material of adherents should be less.
2) Shear compressive and tensile strength of the adhesive
The shear compressive and tensile strength of adhesive indirectly contributes to the load-bearing capacity of the composite material. Thus for high strength of composites material adhesive material, in turn, should have high strength.
3) Porosity and smoothness of contacting surfaces
Porosity is a physical factor that indicates bond strength between surface molecules. If the porosity is high then the bond is low and vice-versa. While high smoothness is a sign of good strength. High smoothness ensures the absence of impurities on any surface. Thus for any contacting surface porosity should be less and smoothness should be as high as possible.
Chemical factors:
1) Degree of polymerization:
The best-suited degree of polymerization which gives strong bond strength differs from adhesive to adhesive. It lies between unpolymerized to a highly polymerized fraction
2) The pH of medium:
The presence of strong acids and alkalies are harmful to adhesive bond strength. Some of the adhesives give better bonding in a slightly acidic medium like vulcanized rubber. On another hand, some adhesives give better bond strength in a slightly alkaline medium like glue.
REFERENCE BOOKS:
1) A textbook of engineering chemistry by M.M. Uppal
2) Applied Chemistry by Krishnamurthy P. Vallinayagam and K. Jeysubramanian TMH Publication
3) A textbook of engineering chemistry by Shashi Chawla
4) A textbook on experiment and calculations in engineering chemistry by S.S. Dara, S. Chand Publication
5) Engineering Chemistry by R.V. Gadag and A.N. Shetty
6) Textbook of polymers science by F.W. Billmer, John Wiley and sons
7) University chemistry, Mahan, Pearson education