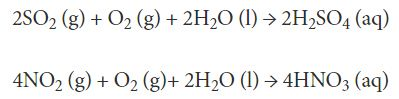Unit-6
Chemistry in the environment
Environmental chemistry focuses on the presence and impact of chemicals in soil, surface water, and groundwater. Environmental chemists study how chemicals - usually contaminants - move through the environment. This is referred to as chemical “fate and transport”. They also study the effects of these contaminants on ecosystems, animals, and human health.Environmental chemistry is the scientific study of the chemical and biochemical phenomenon that occur in natural places. It can be defined as the study of the sources, reactions, transport, effects, and fates of chemical species in the air, soil, and water environments; and the effect of human activity and biological activity on these. Environmental chemistry is an interdisciplinary science that includes atmospheric, aquatic and soil chemistry, as well as heavily relying on analytical chemistry and being related to environmental and other areas of science.
There are four environmental segments: atmosphere, hydrosphere, lithosphere consisting of the abiotic or physical environment, and biosphere—the fourth segment of the environment that consists of flora and fauna. Abiotic and biotic components together constitute the biome environment.
Atmosphere: It is the protective blanket of gases surrounding the earth, which supports life and protects it from the hostile environment of outer space. It absorbs most of the cosmic rays from outer space and a major portion of the electromagnetic radiation from the sun and helps in the heat balance of the earth. It absorbs most of the cosmic rays from outer space and a major portion of electromagnetic radiation from the sun and transmits only near UV, visible & IR radiation by filtering out the harmful radiation below 300nm.
The total mass of the atmosphere: 5 * 105 tones which are 1 millionth of earth's total mass.
Density decreases rapidly with height.
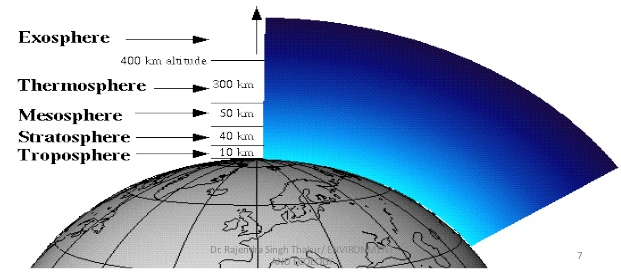
The atmosphere can be divided into the following five concentric layers, depending on the temperature variations:
(i) Troposphere:In this layer, humans and other organisms live.
(ii) Stratosphere:In this layer,the temperature is very low, because of whichtherearenoclouds,dust, orwatervapors.
(iii) Mesosphere:In this layer, the temperature drops to about −95°C. The principal chemical species in the mesosphere are N2, O2, O2+, and NO+.
(iv) Thermosphere or ionosphere:In this layer, most of the gaseous components are ionized under the influence of radiant energy and so the ionosphere contains electrically charged particles such as O+, O2+, and NO+.Radio messages can be transmitted through this layer around the curve of the earth.
(v) Exosphere: In this layer, the temperature is very high due to solar radiation.This region lacks atoms except for hydrogen and helium.
|
Hydrosphere:The range of surface temperature and pressure of our planet permit water to exist in all three states
Solid
Liquid
Gas
Most of the water is contained in the oceans and the high heat capacity of this large volume of water buffers the earth's surface from large temperature changes. Water is the universal solvent and the basis of all life on our planet. Hydrosphere, which covers more than 75% of the earth’s surface, includes all types of water resources—oceans, sea, rivers, lakes, streams, reservoir, glaciers, polar ice caps, and groundwater (that is, water below the earth’s surface). About 97% of the total water available on earth is in the form of oceans, which cannot be used for human consumption owing to their high salt content. About 2% of the water resources are locked in the polar ice caps and glaciers, while only 1% is available as freshwater (surface water—river, lakes, streams, and groundwater) for human consumption and other uses. Freshwater is also available in the form of rain, snow, dew, and so on.
Key Takeaways:
1) It is the protective blanket of gases surrounding the earth, which supports life and protects it from the hostile environment of outer space.
2) Atmosphere absorbs most of the cosmic rays from outer space and a major portion of electromagnetic radiation from the sun and transmits only near UV, visible & IR radiation by filtering out the harmful radiation below 300nm.
3) Hydrosphere, which covers more than 75% of the earth’s surface, includes all types of water resources—oceans, sea, rivers, lakes, streams, reservoir, glaciers, polar ice caps, and groundwater.
4) Biosphere denotes the realm of living organisms and their interaction with the environment, that is, atmosphere, hydrosphere, and lithosphere.
Increased risk of respiratory illness and cardiovascular problems
Increased risk of skin diseases
May increase the risk of cancer
Global warming
Acid rain
Ozone depletion
Hazards to wildlife
Other significant causes of water pollution include:
Dumping solid wastes in water bodies
Disposing untreated industrial sewage into water bodies
Human and animal wastes
Agricultural runoff containing pesticides and fertilizers
The effects of water pollution are very pronounced in our environment.Furthermore, toxic chemicals can bioaccumulate in living beings, and these chemicals can travel their way up the food chain, ultimately reaching humans.
Soil Pollution: Soil pollution, also called soil contamination, refers to the degradation of land due to the presence of chemicals or other man-made substances in the soil. The xenobiotic substances alter the natural composition of soil and affect it negatively. These can drastically impact life directly or indirectly. For instance, any toxic chemicals present in the soil will get absorbed by the plants. Since plants are producers in an environment, it gets passed up through the food chain. Compared to the other types of pollution, the effects of soil pollution are a little more obscured, but their implications are very noticeable.
The preventive measure of soil pollution is:
Reducing chemical fertilizers and pesticide uses.
Recycling is another way to reduce and control soil pollution. Recycling paper, plastics, and other materials reduce the volume of landfills.
Reusing of materials.
De-forestation, the cutting down of the trees, causes soil erosion, pollution, and the loss of fertility in the topsoil. Planting trees or reforestation helps prevents soil erosion and pollution.
Radioactive Pollution: Radioactive pollution occurs when there is a presence or depositions of radioactive materials in the atmosphere or environment, especially where their presence is accidental and when it presents an environmental threat due to radioactive decay. The destruction caused by the radioactive materials is because of the emissions of hazardous ionizing radiation (radioactive decay) like beta or alpha particles, gamma rays, or neurons in the environment where they exist. Since the substances are characterized by radiation – because there is a lot of instability of the particles present in the radioactive materials, it can seriously affect, alter and even destroy the plant, animal, and human life.
Key Takeaways:
1) Any contamination in the air may not only cause many diseases and loss of vision but can also disturb the whole atmospheric system.
2) Air pollution can also cause acid rain which damages the soil, vegetation, and aquatic life of the region.
3) Water pollution is said to occur when toxic pollutants and particulate matter are introduced into water bodies such as lakes, rivers, and seas.
4) The xeno biotic substances alter the natural composition of soil and affect it negatively.
5) Radioactive pollution occurs when there is a presence or depositions of radioactive materials in the atmosphere or environment, especially where their presence is accidental and when it presents an environmental threat due to radioactive decay.
|
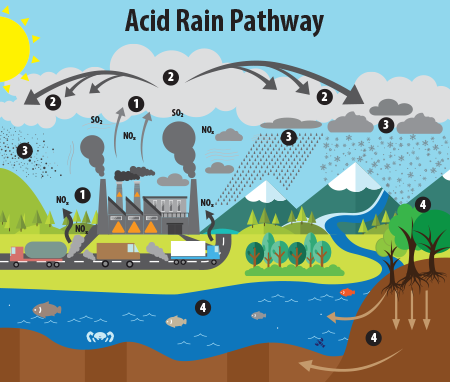
Wet Deposition: This is the most common form of acid rain. Sulfuric acid and nitric acid are the main causes of acid rain. They get mixed with the rain and fall on the earth’s surface as acid rain. They may also precipitate in other forms such as sleet, snow, hail, or even fog. All these are just another form of wet acid deposition via acid rain
Dry Deposition: Now the acids don't need to mix with the moisture and fall as rain. They may also deposit on the earth’s surface in a dry form. This happens more in an area that receives an infrequent and low amount of rainfall, like deserts and arid areas.
|
Key Takeaways:
1) Acid rain is made up of highly acidic water droplets due to air emissions.
2) Wet deposition is any form of precipitation that removes acids from the atmosphere and places them on the surface of the earth.
3) Wet deposition gets mixed with the rain and fall on the earth’s surface as acid rain.
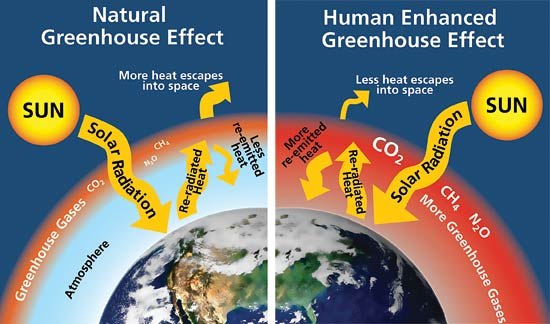
Sunlight passes through the atmosphere and warms the earth’s atmosphere and warms the earth surface. This heat is radiated back toward space. Most of the outgoing heat is absorbed by greenhouse gases (CO2, methane, nitrous oxide, and water vapor) and is re-radiated back to the earth to warm the planet.
“Global warming is a gradual increase in the earth’s temperature generally due to the greenhouse effect caused by increased levels of carbon dioxide, CFCs, and other pollutants. “
Global warming, the phenomenon of increasing average air temperatures near the surface of Earth over the past one to two centuries. Climate scientists have since the mid-20th century gathered detailed observations of various weather phenomena (such as temperatures, precipitation, and storms) and related influences on climate (such as ocean currents and the atmosphere’s chemical composition). These data indicate that Earth’s climate has changed over almost every conceivable timescale since the beginning of geologic time and that the influence of human activities since at least the beginning of the Industrial Revolution has been deeply woven into the very fabric of climate change.
|
Key Takeaways:
1) Global warming is a gradual increase in the earth’s temperature
2) The phenomenon of increasing average air temperatures near the surface of Earth over the past one to two centuries.
3) Sunlight passes through the atmosphere and warms the earth’s atmosphere and warms the earth surface.
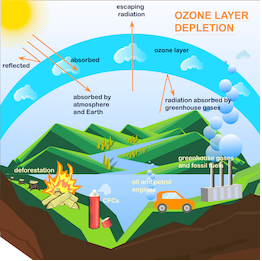
The depletion of the protective O3 layer is because of the presence of particular chemicals in the stratosphere of the earth’s atmosphere. The constant release of compounds like carbon tetrachloride, carbon tetrafluoride, CFCs (chlorofluorocarbon) or freons, and other chlorine or bromine-containing halogens in the atmosphere is the main reason for the depletion. CFC compounds are non-inflammable, non-toxic, nonreactive organic molecules. Hence, it is used in air conditioners, refrigerators, plastic foam production, cleaning computer parts, etc. However, these chemicals mix with normal atmospheric gases and finally reach the stratosphere. Thus, these compounds break down into free chlorine radicals in the presence of powerful UV radiation in the stratosphere.
CF2Cl2 (g) → Cl(g) + CF2Cl(g) The chlorine radicals combine with the stratospheric O3 thereby forming molecular oxygen and chlorine monoxide radicals. Cl(g) + O3(g) →ClO(g) + O2(g) Chlorine monoxide radicals will further react with atomic oxygen to form more chlorine radicals. ClO(g) + O(g) → Cl(g) + O2(g) This process will continue and constantly regenerate chlorine radicals. This, in turn, will lead to the breakdown of ozone.
|
Key Takeaways:
1) The depletion of the protective O3 layer is because of the presence of particular chemicals in the stratosphere of the earth’s atmosphere.
2) CFC compounds are non-inflammable, non-toxic, nonreactive organic molecules which is the main cause of ozone depletion.
3) The constant release of compounds like carbon tetrachloride, carbon tetra-fluoride, CFCs, freons, and other chlorine or bromine-containing halogens in the atmosphere is the main reason for the depletion.
REFERENCE BOOKS:
1) A textbook of engineering chemistry by M.M. Uppal
2) Applied Chemistry by Krishnamurthy P. Vallinayagam and K. Jeysubramanian TMH Publication
3) A textbook of engineering chemistry by Shashi Chawla
4) A textbook on experiment and calculations in engineering chemistry by S.S. Dara, S. Chand Publication
5) Engineering Chemistry by R.V. Gadag and A.N. Shetty
6) Textbook of polymers science by F.W. Billmer, John Wiley and sons
7) University chemistry, Mahan, Pearson education