Units of Hardness
- Parts Per million (ppm): It is defined as the number of parts by weight of CaCO3 equivalent present in per million (106) parts by weight of water.
1 ppm = 1 part of CaCO3 equivalent hardness in (106) parts of water.
- Milligrams per litre (mg/l): It is defined as the number of milligrams of CaCO3 equivalent hardness present in one litre of water.
1 mg/L = 1 mg of CaCO3 equivalent hardness present per litre of water.
It can be easily proved that 1mg/L = 1 ppm, for water
Weight of 1 litre of water = 1kg = 103g = 106mg
= 1000*1000g= 106mg
1mg/L = 1mg of CaCO3 equivalent hardness per 106 parts of water.
1mg/L = 1 part of CaCO3 equivalent hardness per 106 parts of water.
1mg/L = 1ppm
- Degree Clark (0Cl): It is defined as the parts of CaCO3 equivalent hardness per 70,000 parts of water or it is number of grains (1/7000lb) of CaCo3 equivalent hardness per gallon (10lb or 70,000 grains) of water. 10 Clark= 1 part of CaCo3 per 70,000 part of water.
- Degree of French (0Fr): It is defined as the parts of CaCO3 equivalent hardness per Lac (100000) parts of water.
10Fr = 1 part of CaCO3 equivalent hardness per (105) parts of water.
- Milli equivalent per litre (meq/L): It is defined as the number of milli equivalents of hardness present per litre.
1 meq/L = 1 meq of CaCO3 per L of water
= 10-3 * 50g of CaCO3 eq. per litre of water
= 10-3 * 1000 * 50 mg of CaCO3 eq. per litre of water
= 50mg of CaCO3 eq. per litre of water
= 50mg/L of CaCO3 eq. per litre of water = 50 ppm
= 50mg of CaCO3 eq. per 106 litre of water
= 1 mg of CaCO3 eq. per 106 /5 mg litre of water
= 1 part of CaCO3 equivalent per 20,000 parts of water.
1.1.1 Softening
Principle
Water softening refers to the removal of magnesium, calcium and certain other metal cations in hard water, water softening is Water softening is usually accomplished by using lime softening or ion exchange resin, but in recent times the softening is attained with nanofiltration of reverse osmosis.
Reaction
The softening of water is attained by either adding chemicals that form precipitates that are insoluble or by ion exchange method. The chemicals usually added are borax, ammonia, calcium hydroxide (slaked lime) or trisodium phosphate, in the lime soda method, filtration and sedimentation processes are carried out to remove precipitates.
Advantage
- The problems caused by hard water like scaling and excess soap is dealt by water softening.
- The process of water softening also helps in other water treatment process.
- The lime softening process that included high pH can assist in disinfection.
- The softening of water helps to remove manganese, iron and also reduces taste, odour and total dissolved content and also removes radioactivity.
- As water gets stabilized, while recarbonation at the end of the lime water softening, corrosion is avoided in the distribution system.
Limitations
- Lot of studies has not been done to show the effect of magnetic wate r treatment.
- Most of the studies done are not concluded with any effective results.
- Their degree of efficiency fluctuates regularly, depending on water pressure.
- Water is known to revert back in 48 hours’ time, therefore if water remains in the heater for more than two days, the water in the radiator becomes hard.
Comparison of Lime soda and Zeolite process
Sl no | Lime soda process | Zeolite process |
1 | The water treatment plant occupies more area or place
| Water treatment plant occupies less area. |
2 | Water after treatment has lesser dissolved solids
| Water after treatment has much more dissolved solids. |
3 | This method of treatment of water is very economical and the material is also cheap.
| This method of water treatment is expensive, and the material used for softening is also very expensive. |
4 | It cannot operate under pressure
| It can operate under pressure |
5 | This method can be applied to acidic water also
| This method cannot be applied to acidic water |
6 | There is a problem of Settling, coagulation and removal of sludge
| In this method, no problems arise due to coagulation, sludge and settling. |
7 | Residual Hardness is low about 15 to 50 ppm | Residual hardness is low about 10-15ppm |
8 | In order to meet the changes in hardness of incoming water, frequent control and adjustments is required.
| Control test comprises of only testing the hardness of treated water. |
Desalination Process
Desalination is the process that involves generation of fresh water by removing the saline (salt) from the bodies of salt water. There is varying degree of salinity in water that affects the expense of treatment, the level of salinity is measured in parts per million(ppm).
Numerical
- Calculate the amount of lime (85%) and soda (95%) required to soften one million litre of water which contains
CaCO3 = 12.5 ppml, MgCO3 = 8.4 ppml
CaCl2 = ppml, MgCl2 = 9.5 ppml CO2 =33ppml, HCl=Z3ppml organic matter = 16.Bppm
Solution
Impurities(mg/l Multiplication Factor CaCO3 Equivalent(mg/l) Requirement
CaCO3 = 72.5 100/100 12.58 x100/100=12.5 L
MgCO3 = 8.4 100/84 8.4x100/84 =10 2L
CaCl2 = 22.2 100/111 22.2 x100/111 =20 S
MgCl2 = 9.5 100/95 9.5x 100/95 = 10 L+S
CO2 = 33 100/44 33x 100/44 = 75 L
HCl = 7.3 100/73 7.3 x 100/73 = 10 L+S
Nacl does not react with lime and soda
LIME = 74/100 [CaCO3 equivalent of 2 x MgCO3+ CaCO3+ MgCl2+ HCl+ CO2]x

 Volume of water x 100
Volume of water x 100
100 % purity


 74 = [ 2 x 10 + 12.5 + 10 + 10 + 75] x 106 x 100
74 = [ 2 x 10 + 12.5 + 10 + 10 + 75] x 106 x 100
100 1000 85
= 111000 gms.
- The hardness OF 100.000 lit of water was completely removed by passing it through a zeolite softener. The softener then required 40o lit of NaCl containing 100 gm /lit NaCl for regeneration Calculate hardness of water
Solution
Total amount of NaCl required for regeneration = 100 x 103 x 400 = 4 x 107 58.5 gm of NaCl = 50 gm of CaCO3
4 x 107 mg NaCl = 4 x 107/58.5 X 50
CaCO3 equivalent of NaCl =3.419 X 107mg of CaCO3
Volume of water = 100,000 lit
Hardness of water =CaCO3 equivalent of NaCl /hardness
Hardness of water =3.419 X 107 /100,000
Hardness of water =3.419 X 102ppm
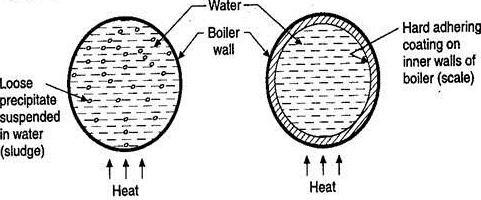
Causes, Effects on boiler operation, methods of prevention
Sludge formation , scale formation
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Sludge :-( formation of sludge )
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Disadvanatages of sludges :-
- They tend to waste some portion of heat.
- Edcessive sludge formation distrub working of boiler and sometimes may choke up the pipe .
Prevention of sludges :-
- Use of water contaning very low quantity of total disolved solids.
- Frequently making blow down opreation i.e replacing salts concentrated water with fresh water.
Scales formation
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
It is caused due to :-
- Decomposition of bicarbonates :-
At high temprature bicarbonates decompose into sticky water insolube material.
Ca ( HcO3)2 ----------------- CaCO3 + H2O + CO2
Mg ( HCo3)2 - - -------------- Mg (Oh)2 + 2CO2
2. Hydrolsis of magnesium salts :-
At higher temprature magnesium salts undergo hydrolysis.
MgCl2 + 2H2O ---------------------- mg ( OH)2 + 2HCl
3. Presence of silica :-
The source of ssilica is ( form) from sand and filter .silica may be in the form of colloidal particles. And it can be deposite as calcium silicate or magnesium silicate as firmly adhering materials.
4. Decreased solublity of CaSO4 :-
CaSo4 has lesser solublity at higher temprature hence at high temprature CaSo4 present in boiler feed water will precipitate as hard scale forming materials.
|
Fig:1 Scale and Sludge formation in Boilers
B] Disadvantages of scale :-
1. waste of fuel :-
Scales are bad conductors of heat and resultes in the reduction of heat transfer to the boiler . higher the thickness of scale greater than the wastage of fuel there by . it has been reported that 0.25 cm . scale would increase fuel consuption by aboyut 2 to 3 percent.
Thickness of scale | 0.325 mm | 0.625mm | 1.25mm | 2.5 mm |
Wastage of fuel | 10 percent | 15 percent | 50 percent | 80 ercent |
3. over heating of boiler :-
Scale being pooe conducter of heat it reduces transfer of heat from boiler to boiler water . to keep the required steam pressure we need to overheat the boiler .
4. boiler saftey :-
due to the scale formation the overheating of boiler is done in order to maintain constant stream supply with required pressure . this overheating makes boiler metal to become soft and weak . this cause distortation of boiler tube and becomes dangerous in morden high pressure boiler .
5. Danger of Explosion :-
When thick scale cracks due to uneven expansions the water come suddenly suddenly in contact with the overhead boiler metal. This cause large amount of steam formation suddenly and sudden high pressure is developed . due to sudden high pressure is devloped . due to sudden high pressure the softer boiler metal may burst with explosion.
C] Removal of scales :-
Scales are removeed from time by different ways.
- By use of suitable chemicals the scale can be dissolved and removed.
- Use of scraper or wire brush for thin scales to remove.
- Thick s ales may br removed by hammer and chisle.
- The thermal shocks technique is used to remove hard brittle scale . in this method empty boiler is heated and cooled by cold water suddenly . while sudden cooling the contracting boiler metal excerts pressure on scale to crack them.
- Blow down opreation used if scales are loosely adhering.
D] prevention of scales :-
It is better to minimize scales formation and reduce the problems in steam generation.
- Use of softened water.
- Adding sodium phosphate to the water
- Frequent blow down operations to remove the scales when they are thin.
- Adding sodium aluminate which can trap the scale forming particles.
- Adding organic chemicals like tannin which forms coating on the scale forming particles . this matter becomes easily removable by blow down operation.
Foaming:
This occurs inside the boiler Foaming is the formation of thick layer of steam bubbles on the top of water surface inside the boiler due to:
- High concentration of Impurities
- Presence of vegetable or animal fats in feed water (Carryover from Oil Heaters)
- Increase in level of dissolved & suspended solids (TDS level)
- Increase in Boiler water level
Prevention of Foaming
To prevent foaming, scum or surface blow down shall be carried out frequently to expel any floating impurities from boiler. Also, no lube oil (or oil) shall be allowed to enter the boiler.
Priming:
Priming is the condition of Boiler in which large amount of water is carried along with steam into the steam line. It is Caused by:
- Excessive Foaming
- Insufficient steam space
- Because of sudden rush of steam (This may happen if steam stop valve is opened suddenly)
Prevention of Priming
- Never keep boiler water level too high &
- Open steam stop valve slowly
Actions to be taken during Priming & Foaming of Boiler
- Scum blows down
- Reduce boiler burner firing rate
- Check weather boiler chemical added in excess?
- Detection of source of contamination present in boiler feed water by oil.
Scale and Sludge formation
Boilers are used for steam generation. When hard water is evaporated, progressively the concentration of dissolved salt is increased. When their saturation points are reached, the dissolved salts of calcium and magnesium along with other soluble impurities are precipitates on the inner walls of boilers and in due course of time adhere to the metal surface in the form of scales and sledges. The sludge is generally form by the substance, which have greater solubility in hot water than cold water. So, sludge is formed in colder parts of boiler.
Hard Scale (Scale)
- Forms in steam boiler
-Has the appearance of a white and brown concentration
-smooth texture
Soft Scale (Sludge)
-Forms in the hot water heaters
-Appears as a thick, brown or black sludge
Caustic Embrittlement
This phenomenon occurs in boilers, the caustic materials accumulate in boiler materials. The phenomena can also be described as the cracking of mild steel riveted boiler plates. The process occurs at a temperature of 200°-250°C resulting in the local deposition of concentrated hydroxide.
In Caustic embrittlement the main things that are focussed are the parts of the boiler like bends, cracks, rivets and joints. At high temperature and pressure sodium hydroxide is formed from residual sodium carbonate which undergoes hydrolysis.
Caustic embrittlement is also known as stress corrosion cracking.
There are many causes of caustic embrittlement, including the combined action of the following three components:
- A susceptible material
- A given chemical species
- Tensile stress
Caustic embrittlement can be prevented through several methods, including:
- Controlling the temperature and potential
- Controlling the stress levels and hardness
- Materials are used that do not crack when placed in a given environment.
- Alkali is avoided wherever necessary
- Softening agents such as sodium sulphates can be used in place of sodium carbonates
- Hairlines can be prevented by adding lignin, tannin or sodium sulphate they also prevent infiltration of sodium hydroxide into the areas
Boiler Corrosion
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the corrosion.
All metals can corrode. Some metals, like pure iron, deteriorate very fast. Stainless steel, and, metals that combines with iron and other alloys, is slower to corrode and is therefore used more efficiently.
All small group of metals, are called the Noble Metals, and show much less reaction than others. As a result, they deteriorate rarely.
The main factors which affect corrosion are
1. The more the metal shows its reactivity, the possibility of the metal getting corrode increases.
2.The corrosion occurs faster when the impurities help in setting up voltaic cell.
3. The rate of corrosion is also affected by the presence of electrolytes in water.
4. The rusting of Iron is increased if the amount of carbon dioxide is present in more amounts in water.
5.The rate of corrosion can however be reduced when the surface of Iron is coated with layers of metal that are actually more active than the Iron itself.
6. An increase in temperature (within a reasonable limit) also increases the rate of corrosion.
The residual salts that are not removed by external methods can be removed by adding some chemicals directly into the boiler water. These chemicals are known as ‘Boiler compounds. This method is known as ‘Internal treatment’
E.g.) Carbonate conditioning, Phosphate conditioning, Calgon conditioning, etc.,
a) Carbonate conditioning: Used for low pressure boilers. Here the salts like CaSO4 are converted to easily removable CaCO3. But sometimes it produces NaOH, CO2 and hence Carbonic acid. So, it is less preferred.
CaSO4 + Na2CO3 ------> CaCO3 + Na2SO4
b) Phosphate conditioning: Used for high pressure boiler. No risk of CO2 liberation.
3CaSO4 + 2 Na3PO4 ---------> Ca3(PO4)2 + 3 Na2SO4
Three types of Phosphate salts are used:
- Na3PO4 Tri sodium Phosphate highly acidic water
- Na2HPO4 Di sodium hydrogen Phosphate slightly acidic water
- NaH2PO4 Sodium di hydrogen phosphate highly alkaline water
c) Calgon conditioning: Calgon is the trade name of sodium hexa meta phosphate- Na2 [ Na4 (PO3)6]. With calcium ions it forms a soluble complex and prevents scale and sludge formation. It is used for high- and low-pressure boilers.
2CaSO4 + Na2[ Na4 (PO3)6] --------> Na2 [Ca2(PO3)6] + 2 Na2SO4
Coagulation:
It has been found that when certain chemicals are added to water an insoluble,
gelatinous, flocculent precipitation is formed. This gelatinous precipitate during its formation and descent through the water absorb and entangle very fine suspended matter and colloidal impurities.
The gelatinous precipitate therefore has the property of removing fine and colloidal particles quickly and completely than by plain sedimentation. These coagulants further have the advantages of removing color, odor and taste from the water.
These coagulants if properly applied are harmless to the public.
Most commonly used coagulants:
- Aluminum sulphate [Al 2. (SO4)3 18H2O]:
It is also called simply as alum. Alum which is available in market, is dirty grey solid in the form of lumps containing about 17% aluminum sulphate. This is the chemical, coagulant which is widely used in water treatment plants. Alum reacts in water in the presence of alkalinity; if natural alkalinity is not present sufficient lime is added
The following chemical reactions take place with the various types of alkalinity:
Al2 (SO4 )3 .18H2O+3Na2CO3 -------> 2Al (OH)3 +3Na2SO4 +2CO2 +15H2O
Al2 (SO4 )3 .18H2O +3Ca (OH)2 ------> 2Al (OH)3 +3CaSO4 +18H2O
Al2 (SO4 )3 .18H2O +3Ca (HCO3) 2 -------> 2Al (OH) 3 +2CaSO4 +6CO 2 +18H2O
The insoluble and colloidal aluminum hydroxide [Al (OH) 3] forms the floe which
removes the fine suspended and colloidal impurities. For best results the pH value of water should be between 6.5 and 8.5. The dose of alum should be 0.03 to 0.13 gm/litre depending on the turbidity of water
- Sodium Aluminate [Na2Al2O3 ]:
This is an alkaline compound. The best grade it contains Al2 O3 , 55%; Na2O3 , 34%; Na2CO3 4.5%; Na (OH), 6.3%. This can be used for treatment very easily in the water having no alkalinity. It reacts very quickly and forms the precipitate of aluminum hydroxide.
Its chemical equations are as follows:
Na2 Al2O3 +CaSO 4 ----> CaAl2O3 +Na2 SO4
Na2 Al2O3 +CaCl 2 -----> CaAl2O3 +2NaCl
Na2 Al2O3+Ca(HCO3 )2 -----> CaAl2O3 +Na2CO3 +CO2 +H2O
- Ferric Coagulants:
Generally ferric chloride (FeCI3), ferric sulphate [Fe2 (SO4 )3] or the mixture of both is used for coagulation purpose.
The various chemical reactions which take place are as follows:
2FeCl3 +3Ca(OH)2 ----> 2Fe(OH)3 +3CaCl2
Fe2 (SO4)3 +3Ca(OH)2 ------>2Fe(OH)3 +3CaSO4
- Chlorinated Copperas
It is a mixture of ferric chloride and ferric sulphate prepared by adding chlorine to a solution of ferrous sulphate in the ratio of 1 part chlorine to 7.9 parts copperas.
6FeSO4 +3Cl2 ----->2Fe(SO4 )3 +2FeCl
Sterilization by UV
UV sterilization is not a new technology, it was discovered as early as in1879, but has found its prominence in the 20th century.
UV sterilization, also is known as UV disinfection or Ultraviolet Germicidal Irradiation (UVGI), is extremely useful. The working principle includes, that they breakdown the individual chemical bonds and structures of DNA, RNA and proteins, resulting in making the microorganism unable to multiply or increase. Thus, when organisms are unable to grow, they are considered as dead, they cannot reproduce within a host and are no longer infectious.
This technology effectiveness depends on the total frequency and energy applied, UV sterilization uses the frequency of UVC to destroy biomolecules, that is affected by the distance from the light source and the length of exposure time.
Ozone
Ozone are also potent germicides and powerful oxidizers apart from being good
disinfectants, ozone is an unstable gas and can kill bacteria and viruses effectively, they are powerful oxidizers, with no build-up immunity or toxic residues. Ozone is most effective disinfectant compared to chlorine and other commonly used disinfectants. ozone is short-lived, they return to their normal form when it breaks. When ozone destroys bacteria or any organic compound, the free atoms liberated attack any foreign particle that are present in air or water, however during this process ozone does not produce any toxic compounds unlike chlorine compounds.
For the decontamination of water or air, ozone is considered the most effective and a safe disinfectant, at the right levels and safety standard they can be used effectively in hospitals, industries and homes.
Chlorine
One of the commonly used disinfectants is Chlorine, it is known to be very effective in deactivating the pathogenic microorganisms, Chlorine is easily applicable, measured and controlled, they are relatively cheap and very persistent in nature.
Chlorine has been used for applications, such as the deactivation of pathogenic
microorganisms in waste water, drinking water and swimming pools.
The mode of action is that chlorine kills bacteria and viruses by breaking the
chemical bonds in their molecules Chlorine kills pathogens such as bacteria and
viruses by breaking the chemical bonds in their molecules. Chlorine can exchange atoms with other, like enzymes in bacteria and other cells. When the enzymes come in contact with chlorine, hydrogen atoms are replaced by chlorine in the molecule, resulting in the change in the shape of the compound and they may also fall apart, as the enzymes lose their activity the bacteria or the cell dies.
Breakpoint chlorination is defined as the point where sufficient chlorine has been added to a quantity of water to enable disinfection. In other words, it is at this point that all the undesirable contaminants are removed from the water. It is at this breakpoint that, all the chlorine that is added to the solution gets consumed by the chemical reactions of the contaminants, that results in water having no free availed chlorine (FAC).
In waste water treatment, breakpoint chlorination involves removing of ammonia from a solution, that changes to an oxidised volatile form. Increased amounts of chlorine residue are produced by adding chlorine to water that contains ammonia or organic matter that contains nitrogen.
This process involves the continuous addition of chlorine to water, till a point where chlorine brings about a suppression in the rate of corrosion successfully., that makes the diffusion process difficult due to rust.
Excessive amount of chlorine causes defragmentation to the human tissues. In a reaction Chlorine can undergo strong oxidation causing hydrogen to separate from water, this hydrogen combines with chlorine to form hydrogen chloride and nascent oxygen, that causes damage in tissues.
Generally, in swimming pools, breakpoint chlorination has a certain amount of free chlorine that is used to fully remove combined chlorine from the pool water. It is necessary to avoid the unsuccessful termination
Key Takeaway
Water conditioning may also be used when referring to water filtration or purification to improve the taste and potability of drinking water. Most water is conditioned for human consumption, but water purification may also be utilized for a variety of other purposes, such as industrial applications.
References:
- BETZ, Handbook on Industrial Water Conditioning
- Water Chemistry by Mark .M Benjamin
- Chemistry of Water Treatment by Samuel D Faust
- Chemistry and Water by Satinder Ahuja