UNIT -3
Materials and Green Chemistry
Portland cement
Portland cement name was coined by Joseph Aspdin in 1824, as the cement resembled the white grey limestone, in terms of colour and quality in the island of Portland.
Raw Materials
- Argillaceous or Silicates of alumina in the form of clays and shales.
- Calcareous or calcium carbonate in the form of limestone, chalk and marl, which is a mixture of clay and calcium carbonate.
The raw materials are mixed in the proportion 2:1, with two parts of calcareous material to one part of argillaceous materials, the materials are then crushed and grounded in ball mills to a dry state or mixed in wet state.
The above constituents that form the raw materials undergo chemical reactions during burning and fusion, and combine to form the following compounds called BOGUE COMPOUNDS.
Compound | Abbreviated designation |
Tricalcium silicate (3CaO.SiO2) | C3S |
Dicalcium silicate (2CaO.SiO2) | C2S |
Tricalcium aluminate (3CaO.Al2O3) | C3A |
Tetra calcium aluminoferrite (4CaO.Al2O3.Fe2O3) | C4AF |
The proportions of the four compounds may vary in several Portland cement. The ultimate strength is supplied by tricalcium silicate and dicalcium silicates, the primary setting of cement is provided by tricalcium aluminate, the tricalcium aluminate hydrates very soon but contributes more to early strength.
The tricalcium aluminate also hydrates quickly and generates a lot of heat but its contribution to the strength is very minute. Tetracalcium alumino-ferrite is inactive comparatively.
All the four compounds generate heat when they are mixed with water, the aluminate produce the maximum heat and the minimum heat is produced by dicalcium silicate.
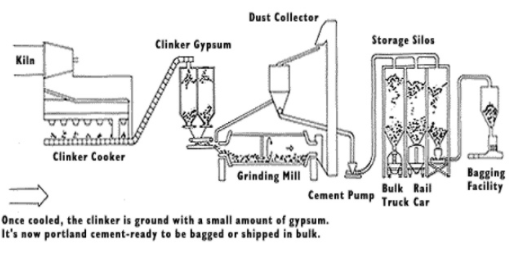
Manufacture and Process Parameters
1. Crushing and grinding of raw material
This is the first step in the manufacture process, in the manufacture of cement. The raw materials are crushed and ground into small suitable size particles. The three types of manufacture process are;
- Dry Process
- Wet Process
The type of manufacturing process decides the crushing and grinding process, in dry process the raw material is dried before crushing.
Wet process
In the wet process, here the limestone is crushed into smaller fragments, it is mixed with clay in a tube mill and water is added to it to make it a slurry, the resulting slurry is kept in tanks and stirred with a rotating arm of supplied with compressed air from the bottom to prevent settling of limestone. The moisture is lost and flakes are formed, and lime, silicas and alumina recombine to form nodular form called the clinker.
Dry process
The raw materials are dried and crushed into a fine powder in the grinding mill. The dry power is further blend and corrected to the right proportion and mixed with the help of compressed air, the resulting powder that is blended and then stored in silos and passed to the granulator comprising an inclined rotating drum or dish.
2. Mixing or Blending
This is the second step where the raw material that is ground(limestone) is mixed with clay. In this step, the grinded raw material (lime stone) is blended or mixed in appropriate proportion (limestone :75%, clay: 25%) and compressed air is employed for mixing and make it into a homogenous mixture, in dry process the homogenous mixture is stored in silos and slurry tanks are used for wet process, the resulting material is called slurry that contains 35-40% of water.
3. Heating
This is the most important step in the manufacture of cement, the slurry formed in previous step is passed through kiln through conveyor belts, initially the mix is preheated to 550OC,during which all the moisture content is evaporated and the clay is broken into silica, aluminium oxide and iron oxide.
In the next zone the temperature is raised to 1500OC, where the oxides form respective silicates, aluminates and ferrite.
2CaO + SiO2 = Ca2SiO4 (declaim silicate (C2S))
3CaO + SiO2 = Ca3SiO5 (tricalcium silicate (C3S))
3CaO + Al2O3 = Ca3Al2O6 (dicalcium aluminate (C2A))
4CaO + Al2O3 + Fe2O3 = Ca4Al2Fe2O10 (tetracalcium aluminoferrite(C4AF))
This forms the final step where the product is cooled to 200O, here the end product obtained in the kiln is known as Cement Clinkers, the appearance of this is greenish black or grey coloured balls.
4. Grinding
A required amount of Gypsum is added in this step to the cement clinkers and grinded into very fine particles that are stored in silos and later packed into cement bags and distributed.
3CaO.Al2O3 + xCaSO4.7H2O = 3CaO.Al2O3.xCaSO4.7H2O
The Expiry date of OPC cement is normally 3 months.
|
Fig 1: The manufacture process of Portland cement.
Role of Microscopic Constituents
Tricalcium Silicate
- This compound hardens rapidly and provides initial strength and is responsible for setting.
- The percentage increase of these silicates provides an early strength of Portland cement to be higher.
- Higher heat of hydration is rendered with high percentage of this compound and shows faster gain in strength.
Dicalcium Silicate
- This compound hardens slowly.
- The effects on strength increases beyond a week.
- They provide long term strength.
Tricalcium Aluminate
- This is the first compound to hydrate and provides strength in the initial days.
- Higher heat of hydration is initiated and provides faster gain in strength
- Upon drying they increase volumetric shrinkage and has poor sulfate resistance
- Low content of Tricalcium Aluminate generates less heat, develop higher strengths and show greater resistance to sulfate attacks.
- They have high heat generation and reacts with soils and water that contain moderate to high sulphate concentrations.
Tetracalcium Aluminoferrite
- Helps in allowing lower temperature in clinkering in cement manufacture.
- Also act as a filler contributes very little strength of concrete even though it hydrates very rapidly.
- Also responsible for grey colour of Ordinary Portland Cement
Setting
The term is used to describe the stiffening of cement paste and mortar or concrete, the setting refers to the state of transformation from liquid state to plastic state and further from plastic state to a solid state. In the setting process, the surface of mortar, concrete or cement becomes rigid adequately and can withstand certain amount of pressure, however some amount of moisture is still present within the mixture, therefore during setting considerable strength is not achieved during cement or motor or concrete, therefore during setting considerable strength is not achieved. Subsequently setting is a stage that achieves stiffness to retain its shape in accordance with the support inside in which it is moulded.
It is divided into Initial setting time and Final setting time
Initial setting time is the time at which cement can be moulded into any shape, without loosing its strength, during this time cement begins to hardens and completely loses its plasticity.
Final setting time is the time when cement loses its plasticity and becomes totally hard and also gains its entire strength is the final setting time of cement.
Hardening
This stage happens after the setting stage, the process helps in gaining strength of s set cement, even during hardening the cement or concrete continue to gain strength, Hardening or stiffening: It happens after setting state. In this stage hydration of silicate compound occurs and thereby gains strength. After the stiffening process, the support is removed and the shape is unaltered without any cracks and deformations.
In other words, the hardened cement gains strength in this stage, once the strength is gained of developed, they are intended to carry loads.
Heat of Hydration
Heat is the main product that helps in binding of cement and water, this heat is given out during the hardening of concrete. This heat has to be taken care of while designing and pouring concrete, during processes such as hardening and curing it has to be managed properly. If proper care of the heat is not taken care then cracking and even structural changes in the concrete can take place. Therefore, it is extremely important to understand hydration of heat and its effects on concrete.
The chemical process of heat of hydration
The process of hydration occurs when cement and water mix together, hydration is not just the binding that takes place in the cement, but occurs when two molecules bind together there is loss of moss resulting in the release of energy. In this process the binding of molecules results in an exothermic chemical reaction. This is called the heat of hydration.
Soundness
The soundness of cement, refers to the ability of cement, to retain its volume after it is hardened, in other words the cement should undergo a minimal change in its volume after it is hardened.
The various properties of cement mainly influence the properties of hardened concrete or mortar. Generally, once the concrete or mortar gets hardened, they do not go under expansion or contraction. i.e., the volume of concrete or mortar does not change, once they are set.
In cases where there is change in the volume of cement, the cement may expand or contract and development of cracks can be expected, such a cement is called unsound cement. Unsound cement causes numerous problems to the durability of structure.
Characteristics
- Presence of Excess lim
The soundness of the cement is affected by the presence of excess lime. The presence of lime shows the formation of slaked lime as they hydrate very slowly, the slaked lime is known to occupy a larger volume than the free CaO, the properties of hardened concrete is affected due to the slow hydration process of lime. The difference in the rate of hydration of lime and slaked lime leads to change in the volume of hardened concrete. Therefore, a limit has been set in the ordinary Portland cement regarding the presence of free lime & magnesia in cement content.
- Presence of excess magnesium oxide
The rate of hydration is affected, due to the presence of magnesium oxide (MgO), MgO reacts with water in a similar manner and forms an unsound cement.
- Inadequate burning
In the manufacture of cement, the raw materials are fed inside the Kiln, during this process, various raw materials like lime and other acidic oxides gets mixed inside the kiln, if there is improper burning and cooling process, then it leads to presence of excess lime, resulting in the formation of further unsound cement.
Excess Calcium Sulphate
The next compound that causes expansion is calcium sulphate (CaSo4). In the later stages of cement manufacture gypsum is added to the cement clincher to prevent flash setting of cement, bit if the added gypsum is in excess it reacts withC3A and forms calcium sulfoaluminate during setting., the compound formed leads to the expansion of the hardened concrete, hence the amount of gypsum to be added, the standard limit should be followed, these limits are decided to bring soundness of cement.
Applications
- Cements are usually mixed with an inert material known as aggregate, cements are usually used alone but are more often used in mortar and concert.
- Mortar is cement mixed with sand or crushed stone that may be around 5mm, mortars are used for binding bricks, blocks, and stone in walls or as surface rending
- Concrete is a mixture of cement, sand or other fine and coarse aggregate and has a size up to 19 to 25 mm (0.75 to 1 inch) in size, but the coarse aggregate may also be as large as 150 mm (6 inches) when concrete is placed in large masses such as as dams, concretes are used in a range of construction purposes.
- The bases used in roads are mainly mixture of soil and Portland cement. Portland cement also is used in the manufacture of bricks, tiles, shingles, pipes, beams, railroad ties, and various extruded products. The products are prefabricated in factories and supplied ready for installation.
High Alumina Cement
These are organic materials, that usually form a dense texture when they react with water. They are known to have excellent refractory, rapid hardening property and resistant to chemical attacks. Bauxite is an aluminium ore; clinkers are formed by bauxite and lime when these clinkers are subjected to grinding this type of cement is produced. The percent of the total alumina content present should not be less than 32 and the ration by weight of alumina to that of lime is between 0.85 and 1.30.
Characteristics of High Alumina Cement
The characteristics of this cement can be summarized in following points:
- They have a low pH and harden rapidly
- It has high durability in sulphuric acid and has high refractoriness
- The alumina is less reactant than lime or alumina
- They form ceramic bonds at high temperatures, and also act as binding agents when added to refractories
- It has high resistance to chemical corrosion. So, it is widely used also in construction of water pipes, sewage pipes, factory drains, coastal constructions and in factory chimneys.
White Cement
White cement is similar to that of grey Portland cement, bit differs in its colour and finess. The white colour of this cement is determined by the raw materials used and the manufacturing process. This cement allows a wide range of colour options for producing architectural and structural concrete
This cement is made from raw materials that have little or show absence of iron or manganese, in general china clay is used together with lime stone or chalk.
White cement is more expensive and costly than the ordinary portland cement because of the manufacturing process and the raw material used.the compressive strength of white cement is less compared to portland cement.
Rapid Hardening
They are also known as high early strength cement. The lime content forms the major difference between the Rapid hardening cement and the Portland cement. Large amount of lime forms distinguishing feature.
The curing period of rapid hardening cement is less, it is very economical.
They show good resistance to sulphur and shrinkage during curing and hardening of cement is less.
Strength is gained in relatively a very short period, and a good speed in construction can be achieved.
Ready Mix Concrete.
Concrete forms the major component for many multi-storeyed buildings. The quantity and quality of concrete shows direct strength and durability to the structure. In this case RMC has much relevance.
Few things become more important to produce than concrete in worksites. In work sites bags of cement, gravel, sand and other additives should be bought to the construction area, water also becomes a necessary factor, and also a concrete mixing hooper them is rented. When the ingredients are mixed and loaded into the hooper a minute change in the dry/wet ratio can cause the entire batch of the concrete to be wasted. To overcome this rather messy and time-consuming condition. Ready mix concrete is introduced.
RMC is a special material that aggravates the cement whereas the other materials are weighed and mixed at the plant using a central mixer or a truck mixer and then brought to the construction site, and can be readily used by the builders the fresh concrete is manufacture away from the site and transported to the site within the requisite time. Ready mix refers to concrete that is batched for delivery from a central plant instead of being mixed on the job site. Each batch of ready-mixed concrete is tailor-made according to the specifics of the contractor and is delivered to the contractor in a plastic condition, usually in the cylindrical trucks often known as "cement mixers."
Flyash is a fine powder that is obtained as a by-product that is formed by burning pulverised coal in electric generation power plants. It is a pozzolan and contains aluminous and siliceous material that form cement with water, when fly ash is mixed with water and limethey form a similar compound of Portland cement. Thus, fly ash forms a suitable material in blended cement, hollow blocks tiles and other building material, fly ash improves the strength in concrete mixes and makes it easier to pump.
Properties of Fly Ash
Fineness of Fly Ash
As per ASTM, the fly ash fineness is checked in both wet and dry sieving the percentage of fineness is measured when sample is passed through a sieve and the amount that is retained on a 45-micron sieve determine the fineness of the fly ash. Further fineness is also measured by the LeChatelier method and Blaine Specific Surface method.
Specific Gravity of Fly Ash
It shows a low value of 1.90of sun-bituminous ash to a high value of 2.96 of specific gravity in iron rich bituminous ash.
Size and Shape of Fly Ash
They are spherical in shape as it is a very fine material the size ranges from 10 to 100 microns.
Colour
The colour mainly depends on the chemical and mineral components, a dark grey to black colour is due to an unburnt content, brown colour is due to the presence of iron content and light and tan colour is due to the lime content.
Applications of Fly ash
- They show great potential for capture, utilization and storage of CO2 applications.
- Adsorbents of fly ash show more better performance than commercial adsorbents, when applies in CO2 capture.
- Carbon utilization that uses fly ash produce valuable products.
- New pathways that use fly ash for carbon utilization are being introduced.
- Advantages
- Compared to Portland, fly ash is cost effective and is more environmentally friendly as it is a by-product of low embodied energy.:
- They are resistant to cold weather and produce various set time
- Depending upon their use they show their strength, they also reduce cracks, permeability and bleeding. High strength gains.
- Can be used as an admixture
- It is a non shrink material and shows great workability.
- Produces dense concrete with a smooth surface and sharp detail
- Allows a low ratio of water-cement ratio for same slumps as compared to other no-fly-ash mixes.
- Reduces heat of hydration and carbon emissions.
Disadvantages
- Small builders and contractors may not be familiar with the fly ash, that can have different properties on how and where they are procured.
- fly ash has the tendency to lose effloresce and other concerns include its nature to freeze and thaw, with these features they may face rejection from builders
- It gains strength gradually
- Fly ash is limited to seasons and have lower strength gain
- Increased need for air-entraining admixtures
- Increase of salt scaling produced by higher proportions of fly ash
Introduction
Green chemistry also known as sustainable chemistry, is a branch of chemistry that deals with the development and design of processes and products in order to reduce or completely remove the produce of toxic substances. They are not same as the environmental chemistry. Green chemistry mainly focusses on the production of particles that are sustainable and environment friendly like non-renewable resources and methods to control the environment.
The Principles of Green Chemistry
The twelve principles illustrated in the year 1988 by the American chemists’ Paul Anastas and John Warner to lays the foundation for green chemistry are given below:
- Prevention of waste: Once the waste is generated, it is always preferred to be cleaned up thus preventing the formation of waste products.
- Avoiding the generation of hazardous chemicals: In industries the toxic and hazardous chemicals that are involved in various processes and reactions pose a threat to human health, this must be optimized to generate such toxic substances
- Atom economy: Any process when taken into consideration involves some amount of wastage. Through green chemistry all, the synthetic processes and methods need to consume and incorporate all the raw materials so that the final product is obtained with minimal wastage. This concept however has to be strictly followed
- The design of safe chemicals: when chemical products are prepared for various chemical processes care should be taken to make the chemical environmentally friendly and also nontoxic to humans.
- Design of safe auxiliaries and solvents: The use of solvents and auxiliary is a common feature in any process, however the use of these should be minimized for all process and if they are used, the usage should be less and non-hazardous in every possible way.
- Energy efficiency: The energy used for various processes should be minimised and the available energy usage should be minimal.
- Incorporation of renewable feedstock: in the processes that are involved in chemical analysis, the usage of raw materials and feedstock should be of renewable variety they must always be preferred over the non-renewable materials.
- Reduction in the generation of derivatives: The derivatives that are not necessary during process, must be minimized as they use plenty of reagents and chemicals that results in excess waste of chemicals.
- Incorporation of Catalysis: In chemical processes, the energy required for the processes and chemical reactions should be reduced and the use of chemical catalysts and catalytic reagents must be introduced.
- Designing the chemicals for degradation: Degradation of chemicals is an important feature as care has to be taken care when a chemical is being prepare for any specific fiction, they should be environmentally friendly and the chemical breaks down into nontoxic chemicals.
- Incorporating real-time analysis: The real time analysis of any product or any process should be monitored to ensure any toxic substances are produced during the process, various methodologies should be implemented that real time data is available for monitoring the process, this helps to stop and dangerous substances during any stage of the process
- Incorporation of safe chemistry for the prevention of accidents: All the chemicals prepared should be safe and should not cause any accidents during the workplace as explosion and fire can occur if the substances are not carefully prepared, chemicals used should be therefore help to develop a safer environment for the process to take place in.
Concept of Carbon Credits
A carbon credit is a permit that consents to the company that holds it to emit a certain amount of carbon dioxide or other greenhouse gases. One credit permits the emission of a mass equal to one ton of carbon dioxide.
Companies that are involved in pollution permits are allowed to continue to pollute up to a certain limit. The limits are periodically reduced, the company can also sell the unneeded credits to another company that may need them.
The ultimate goal of carbon credit is to reduce the emission of greenhouse gases into the atmosphere. However private companies are warned to reduce the emissions and they are also fined if they exceed the limits specified, they can make money by saving and reselling some of their emissions allowances. Companies get a set number of credits, which decline over time. They can sell any excess to another company.
Key take away
The importance of cement and its manufacturing the various other forms of cementing material are highlighted with their importance and contribution.
Green chemistry mainly focusses on the production of particles that are sustainable and environment friendly like non-renewable resources and methods to control the environment. Carbon credits were devised as a market-oriented mechanism to reduce greenhouse gas emissions.
References:
1.Green Chemistry A Textbook by V.K Ahluwalia
2.Green Chemistry Fundamentals and Applications by Suresh.C. Ameta, Rakshit Ameta.
3.Durability of Concrete by Mark alexander and Arnon Bentour
4.Undestanding Cement by Nicholas B