Fuel is a material which can react with other substance to release energy in the form of heat energy, the energy is otherwise used to do work. Initially this concept was applied only to those materials that are able to produce chemical energy, but has now applied to other sources of heat energy like nuclear energy.
A fuel is a substance that contains carbon and hydrogen undergoes combustion in presence of oxygen to gives large amount of energy.
 Fuel + O2 CO2 + H2O + Energy
Fuel + O2 CO2 + H2O + Energy
Types of calorific value (Higher and lower)
Higher Calorific Value
- Gross calorific value of a fuel can be defined as the total amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel (S T P) and on cooling the products of combustion to 15-degree c. The gross calorific value is also called as higher calorific value.
- The G.V.C is of only theoretical importance because in actual practice we do not have any provision of cooling the products of combustion during combustion of fuel in an engine, furnace or any other fuel burning device.
Lower Calorific Value:
- Net calorific value is defined as the amount of heat obtained practically on complete combustion of unit mass of solid or liquid fuel or unit mass of solid or liquid fuel or unit volume of a gaseous fuel at step and the products of combustion are allowed to escape with some heat N.C.V is also called as lower calorific value.
Determination of calorific value by bomb calorimeter and Boy’s calorimeter
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter. (If the liquid is volatile, then it is filled in a polythene capsule of negligible mass then used in experiment.
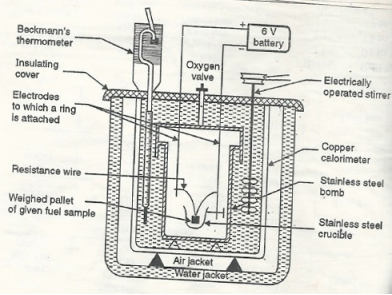
Construction: A bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot: -
It is a cylindrical strong stainless-steel pot having a lid. the lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel. there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter: -
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter, which can record the rise in temperature of welter due to absorb in a heat generated.
- There are insulator stands between calorimeter and water jacket.
Accessories: -
- There is a pellet press to convert the powder of solid fuel to pellet form. for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
|
Fig: Bomb Caloriemeter
Working: -
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible. keep the crucible in the ring of the electrode. keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs. keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water (t1° c).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5. these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 (along with liberation little heat).
- Note the maximum temperature reached. after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Principle: -
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
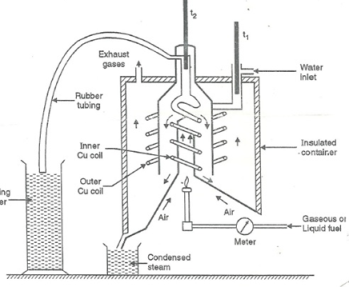
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
- Gas burner
- Combustion chamber (chimney)
- Thermometers
- Insulating cover
- Gas burner: -
- There is a gas burner in which a known volume of gas is burnt at a known pressure. the gas is burnt at the rate of 3 – 4 lit. per minute.
3. Combustion chamber (chimney): -
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of its water enters from top of the outer coils, moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer: -
There are two thermometers to measure temperatures of inlet water and outlet water.
5. Insulating cover: -
The assembly is covered with by an insulator to detach combustion chamber from atmosphere. there are three holes for exhaust gas, water inlet and condensed steam.
Working: -
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady (temperature in and around the combustion chamber) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water (t2 – t1)
|
Fig: Boy’s gas calorimeter
Calculations: -
- First convert the volume of gas burnt to volume of gas at STP. let this STP volume be V
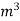 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
(assuming no heat loss in the steady state conditions)
Vl = w (t2 – t1)
L = 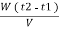 Kcal /
Kcal / 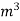
The mass of condensate water per m of gas will be m/v kg /m.
If this water had left as steam in product gases it would have taken away heat.
= 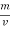 *587 kcal /
*587 kcal / 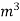
Therefore,
NCV = GCV - 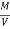 *587
*587
NCV = 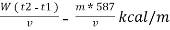
Numerical problems on Bomb and Boy’s calorimeter
A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol.
Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself.
Calculate the heat absorbed by the water (qwater)
m = 1200 grams
cwater = 4.184 J/gºC
∆T = 33.20 – 25.00 = 8.20ºC
qwater = (m)(c)(∆T), so
qwater = (1200 g)( .4 184)( .8 20 C)
=41170.56 J
= 41.2 kJ ⋅
Calculate the heat absorbed by the calorimeter (qcal)
The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
Ccal = 837 J/ºC
∆T = 33.20 – 25.00 = 8.20ºC
qcal = (Ccal)(∆T), so,
qcal = (837 )( .8 20 C) = 6863 J 4. = 6.86 k
Proximate and Ultimate Analysis
The key difference between proximate and ultimate analysis of coal is that proximate analysis is the technique used to analyse the moisture content, ash content and fixed carbon of coal whereas ultimate analysis is the technique used to analyse the chemical composition of coal.
The technique of proximate analysis involves the determination of the different compounds present in a mixture. Ultimate analysis, on the other hand, involves the determination of the number and types of different chemical elements present in a particular compound. Therefore, these two techniques are related to each other.
Proximate analysis of coal is the process of determining the presence of different compounds and their amounts in coal. The technique of proximate analysis was developed by Henneberg and Stohmann (German scientists) in 1860. This analysis technique involves the partitioning of compounds into different categories depending on the chemical properties of these compounds. Mainly, there are six categories of compounds as moisture, ash, crude protein, crude lipid, crude fibre, and nitrogen-free extracts. In the process of proximate analysis of coal, the moisture content of coal, ash content of coal and the fixed carbon content of coal are determined.
The ultimate analysis of coal is the process of determining different chemical elements present in coal. This technique allows us to get more comprehensive results compared to the proximate analysis process.
In this analysis technique, we test moisture, ash, carbon, hydrogen, nitrogen, sulphur and oxygen content of the sample to determine the elemental composition of the coal sample. Therefore, each and every chemical element in the sample is analysed through chemical routes and then we can express the contents as percentages with respect to the total mass of the sample. Mostly, this analysis technique is useful in the coal and coke industry.
Dulong’s formula for calorific value. (Numerical problems on calorific value.)
The calorific value of a fuel can be theoretically determined by using Dulong’s formula. It is assumed that heat evolved comes from the combustion of carbon, hydrogen, and sulphur present in the fuel. The total heat evolved is equal of heat evolved by the combustion of individual constituent’s present.
The calorific value of hydrogen = 34500 cal/gm.
Calorific value of carbon = 8080 cal/gm
Calorific value of sulphur = 2240 cal/gm.
Then, Dulong’s formula is HCV =
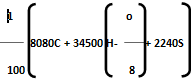
Where, C, H, O, S are percentage fractional weight of carbon, hydrogen, oxygen and sulphur respectively obtained from the analysis of 1 gm of the fuel.
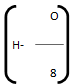 represents the available hydrogen.
represents the available hydrogen.
It is assumed that oxygen present in fuel combines with hydrogen to form water. 8 parts by weight of oxygen combines with one part by weight of hydrogen, to form water. So actual heating value of hydrogen is obtained by above expression.
Numerical problem :
A sample of coal has following composition by mass C = 70 %, O = 8 %, H = 10 %, N = 3 %, S = 2%, Ash = 7 %. Calculate H.C.V. and L.C.V. using Dulong formula [Difficulty level-high]
Solution: HCV = 1/100[8080 C+34500(H-O/8) +2240 S] = 1/100[8080 + 70+34500(10-8/8) +2240 X 2] = 1/100 [565600 + 34500 (10-1) X 4480] = 1/100 [565600 + 310500 + 4480] = 1/100 [880580] = 8805.80kcal/kg LCV = [ HCV- 9/100 H X 587] kcal/kg = [8805.80-9/100 x 587] = [8805.80- 528.30] = 8277.80 kcal/kg
Answer: HCV/GCV = 8805.80kcal/kg
LCV/NCV = 8277.80 kcal/kg
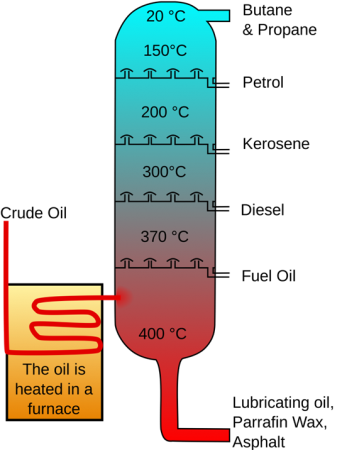
Refining of crude oil
In an oil refinery the crude oil is converted into valuable goods such as petroleum, naphtha, gasoline liquified gas, kerosine etc. Oil refineries are usually huge, vast industrial facilities with extensive pipelines running throughout, holding fluid streams in between.
- Many substances are present in petroleum as a mixture, they include lubricating oil, paraffin wax, diesel, gas, wax etc.
- Therefore, it becomes important to separate these substances and use them separately for different purposes, hence they are refined and the process of separation various constituents of petroleum is called petroleum refining.
- It involves a three-step process and usually performed in oil refineries.
- The first step is distillation process, that separates the crude oil from various components. The lighter constituents rise up as vapour or remain as a liquid while the heavier constituents remain at the bottom.
- In the next step the heavier constituents are converted into gas, gasoline and diesel.
- The last step is to remove the impurities in the constituents and obtain the products in pure form. The most common method of separating components into their constituents is by fractional distillation when due to boiling differences in temperature the crude oil heat up, spray and then condense the vapour.
- New methods, in a method called conversion, use Chemical processing on certain fractions to produce others. For example, chemical processing may split lengthier chains into shorter chains. This allows a refinery to convert diesel fuel into gasoline, depending on the gasoline demand.
|
Fig 1:Crude Oil Distillation
Knocking, this usually occurs in an internal combustion engine, where sharp sounds occurs due to the premature combustion from the part of the compressed air-fuel mixture present in the cylinder. When an engine functions smoothly in the combustion chamber, the charge burns with the flame front smoothly progressing from the point of ignition. In some cases, depending upon the composition of the fuel, at high compression ratios, some of the charge may ignite spontaneously ahead of the flame front resulting in burning in an uncontrolled manner that produces pressure waves of high frequency. The waves are responsible for forcing parts of the engine to vibrate and produce audible sound.
The effects of Knocking can cause overheating of the spark-plug points, erosion of the combustion chamber surface, and rough, inefficient operation.
Octane and Cetane numbers
Octane Number and Cetane Number are the standards to measure the tendency of fuel to ignite spontaneously. The performance of gasoline is measured by the Octane number on the other hand the cetane number measures the performance of diesel. The reason why petrol can’t be used in diesel engine and diesel can’t be used in a petrol engine is that when the fuel that has high octane number will have low cetane number and high cetane number fuel has low octane number.
As per the Standard operating conditions the Octane number of a fuel defines the percentage of Iso-butene present in a mixture of Iso-octane and heptane. When used in a gasoline engine the Octane rating signifies the ability to resist auto ignition. As air and fuel are compressed together, gasoline tends to ignite a spark at the end of compression by a spark plug. If gasoline with low octane number is used it creates problems during ignition and tends to adapt to auto combustion easily due to excess of heat and pressure effects on the other hand, fuels that have high octane value takes more time to burn but provides maximum efficiency to the gasoline engine.
Cetane is a type of chemical compound known as a Hexadecane. Cetane number is opposite to octane number, and measures how quickly the engine burns inside a compressed engine. Cetane compounds tend to ignite spontaneously under compression, therefore they are assigned as cetane number of hundred. Cetane number of a fuel can be defined as the percentage volume of n-hexadecane in the mixture of n-hexadecane and 1-methylnaphthalene which is responsible to provide ignition delay period.
Doping Agents
An antiknock agent is a gasoline additive that is used to reduce knocking that occurs in engines, the fuels octane number is increased by increasing the temperature and pressure at which auto-ignition occurs. As the gasoline engine developed, gasoline and the engine were harmonized to attain the best possible matching of characteristics. One of the important characteristics of gasoline is it is volatile in nature and antiknock quality, Volatility is a measure of the ease of vaporization of gasoline, Gasoline also helps to start engines in cold weather and to avoid vapour lock in hot weather conditions.
Antioxidants
An antioxidant are molecules that are able to inhibit the oxidation of other molecules. Oxidation involves a chemical reaction where electron or hydrogen from a substance moves to an oxidizing agent, the reaction produces free radicles that can initiate chain reactions.
Anti-icing
Fuels posses’ properties that make it difficult to store them and transport fuels for longer periods, fuel additives are compounds that are soluble and affect the properties of fuel, one on the most important additive in Anti-icing. They are mainly fluid base known as monoethylene glycol and additives. The air temperature at very high altitudes drops to minus 60 and 70 degree. The additives that are used in anti-icing form useful tools that help prevent clogging of fuel transmission lines and freezing of storage tanks in cars and aircrafts.
When aircrafts are at a high altitude, there is decrease in temperature during which the dissolved water turns into ice or superheated liquid. Extreme cold water becomes ice when they come in contact with pipes or filters eventually causing clogged paths.
These additives have low solubility to fuel and high solubility towards water, this property becomes effective when the dissolved water is separated from the fuel additive quickly reach the water and dissolve into them thus preventing the freezing by reducing the freezing point of water.
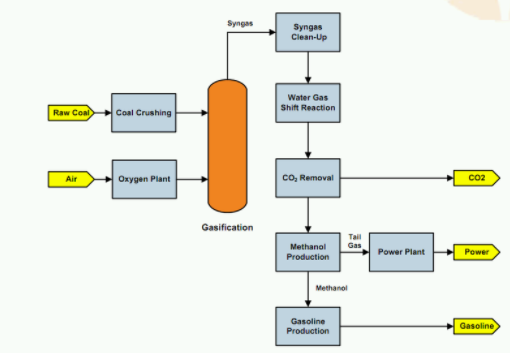
Fischer-Tropsch Process
It is a process for the preparation of gasoline from a non-petroleum origin, the process involves
- The H2 and water gas are passes through the Fe2O2 tower where the H2S that is present as an impurity is removed.
- They are then passed through Fe2O2 and N2CO2 tower, here the organic sulfur is removed.
- The resulting gases are compressed to 5-25 atmps and passed through catalyst bed, these catalyst beds have a ratio200:0C, the catalyst bed is made up of cobalt: thoria: magnesia: kieselguhr earth present in the ratio 100:5:8:200.
- The gaseous mixture that is thus formed are sent through coolers, and a liquid resembling crude oil is obtained.
- The crude oil thus formed are passed through fractional distillation in a fractionating column, where gasoline and heavy oil is formed.
- Heavy oil is again used for cracking purposes
- The quality of the product formed depends on the Fischer tropic catalysts pressure and temperature
|
Fig 2: Process over view
Catalytic Cracking
In this process large hydrocarbon molecules are broken into smaller and more useful molecules, the reaction takes place in the presence of a catalyst, that promotes breakage of large molecules in a way that forms gasoline as maximum yield. The feed in the catalytic reaction is gas oil or naphtha, the residue formed in the catalytic reaction is recycled back into the feed stream. This recycling is called recycling of extinction.
Reaction
The first step is the cracking reaction, where the hydrocarbon feed combines with the catalyst at 900F and 482 OC, the cracking reaction takes place and coke is formed, the coke that is formed coats the catalyst preventing the catalyst from burning off and promote the cracking reaction. Renewal of the catalyst takes place by burning off the coke deposits from the catalyst surface during regeneration. The resulting hydrocarbons are separated using conventional fractionating equipment.
Advantages
- Catalytic cracking is a controlled process
- The reaction takes place at low temperatures and pressure (3000C-400OC) and 1-5kg/cm2
- The catalytic activity produces a higher yield of branched-chain, unsaturated and aromatic hydrocarbons
- Sulphur content of products is low
- Product yield and octane numbers are high.
The common plants used for biodiesel production is Soybean and oil palm. Microorganisms such as algae and bacteria also form sources of biodiesel, they seem promising but are economically difficult to develop Few species of algae have up to 40% lipids by weight that can be converted to synthetic petroleum or biodiesel.it is estimated that few algae and cyanobacteria yield between 10 and 100 times more fuel per unit area than plant-based biofuels.
Is a fuel where the chemical energy stored in the fuel is converted into mechanical energy, any internal combustion engine, where air is compressed to very high temperatures to ignite the diesel fuel injected into the cylinder. The piston is actuated by combustion and expansion, the mechanical energy can to use to drive freight expansion, The tractors and marine vessels. A limited number of automobiles also are diesel-powered, as are some electric-power generator sets.
Applications of CNG
- Widely used in power generation
- Used for Domestic purposes.
- It is used in transportation sector
- Used in manufacture if fertilizers.
Advantages of CNG
- The octane number is 110, therefore it forms an excellent spark ignition engine fuels, due to the high-octane number flame speed is high and can operate with high compression rates.
- They have low engine emissions and aldehyde emission is lower than alcohols
- There are present naturally in abundance and can be produced from Coal
- It is environmentally friendly
- It is Safer and easier to store, and also reliable
- It’s less expensive than other fossil fuel energy sources
- It Minimizes dependency on foreign oil
Disadvantages of CNG
- Low energy density shows poor engine performance.
- It has Low engine volumetric efficiency because of gaseous fuel
- It Needs Large storage tanks, which is a safety concern
- It has Inconsistent fuel properties
- Its Refuelling is a slow process
- It’s highly combustible
- It is Non-renewable energy source
- It emits some quantity of greenhouse gases
Key takeaway
- A fuel is any material that can be made to react with other substances so that it releases energy as heat energy or to be used for work.
- The non-new able fuels, which include coal, oil, and natural gas, supply about 80 % of the world’s energy. They provide electricity, heat, and transportation, while also feeding the processes that make a huge range of products
References
1.Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
2. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
3. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand49. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
4. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand