Unit I
General Chemistry
Content
The arrangement of atoms in the tabular form for identification of their properties such as their atomic number, atomic weight, electronic configuration and other chemical properties. The elements are arranged in periods and groups form. The seven row that exist in the periodic table are called as the periods that contains elements with similar behaviour. Six groups have accepted names as well as assigned numbers: for example, group 17 elements are the halogens; and group 18 is the noble gases. Also displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals.
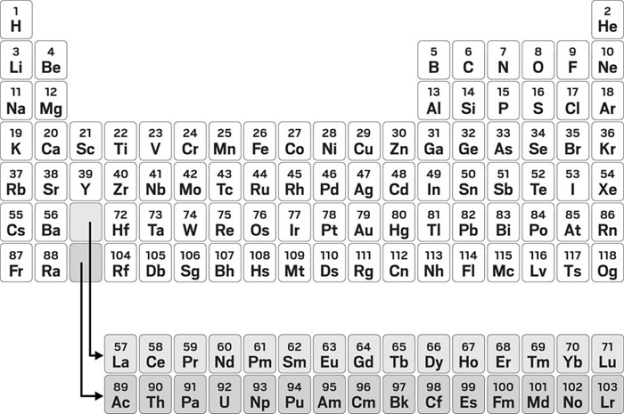
Each chemical element has a unique atomic number (Z) representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent has seven neutrons, and a very small fraction have eight neutrons. Isotopes are never separated in the periodic table; they are always grouped together under a single element. Elements with no stable isotopes have the atomic masses of their most stable isotopes, where such masses are shown, listed in parentheses.
The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:
Zeff = Z – S
Where Z is the atomic number and S is the number of shielding electrons.
Higher energy electrons can have other lower energy electrons between the electron and the nucleus, effectively lowering the positive charge experienced by the high energy electron.
The shielding effect is the name given to the balance between the attraction between valence electrons and protons and the repulsion between valence and inner electrons. The shielding effect explains the trend in atomic size on the periodic table and also why valence electrons are readily removed from an atom.
Penetration describes the proximity to which an electron can approach to the nucleus. In a multi-electron system, electron penetration is defined by an electron's relative electron density near the nucleus of an atom. Electrons in different orbitals have different wave functions and therefore different radial distributions and probabilities. In other words, penetration depends on the shell and subshell. For example, we see that since a 2s electron has more electron density near the nucleus than a 2p electron, it is penetrating the nucleus of the atom more than the 2p electron. The penetration power of an electron, in a multi-electron atom, is dependent on the values of both the shell and subshell.
Within the same shell value, the penetrating power of an electron follows this trend in subshells:
s>p>d>f
And for different values of shell and subshell, penetrating power of an electron follows this trend:
1s>2s>2p>3s>3p>4s>3d>4p>5s>4d>5p>6s>4f
And the energy of an electron for each shell and subshell goes as follows...
1s<2s<2p<3s<3p<4s<3d<4p
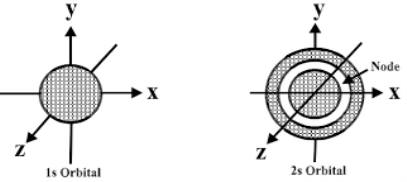
The electron probability density for s-orbitals is highest in the center of the orbital, or at the nucleus. If we imagine a dartboard that represents the circular shape of the s-orbital and if the darts landed in correlation to the probability to where and electron would be found, the greatest dart density would be at the 50 points region but most of the darts would be at the 30 point region. When considering the 1s-orbital, the spherical shell of 53 pm is represented by the 30 point ring.
Electrons which experience greater penetration experience stronger attraction to the nucleus, less shielding, and therefore experience a larger Effective Nuclear Charge, but shield other electrons more effectively.
Shape of s orbital:
The s orbital is spherical having the nucleus at its centre which in two dimensions and can be seen as a circle. The s-orbitals are spherically symmetric having the probability of finding the electron at a given distance equal in all the directions. The size of the s orbital is also found to increase with the increase in the value of the principal quantum number, thus, 4s > 3s> 2s > 1s.
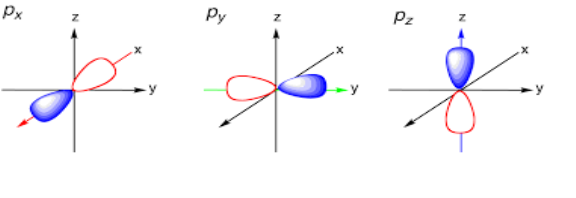
Shape of p orbital:
Each p orbital consists of two sections that is known as lobes which lie on either side of the plane passing through the center that is nucleus. The three p orbitals differ in the way lobes are oriented whereas they are identical in terms of size shape and energy. The lobes lie along one of the x, y or z-axis, these three orbitals are given the designations 2px, 2py, and 2pz. Thus, we can say that there are three p orbitals whose axes are mutually perpendicular. Similar to s orbitals, size, and energy of p orbitals increases with an increase in the principal quantum number (4p > 3p > 2p).
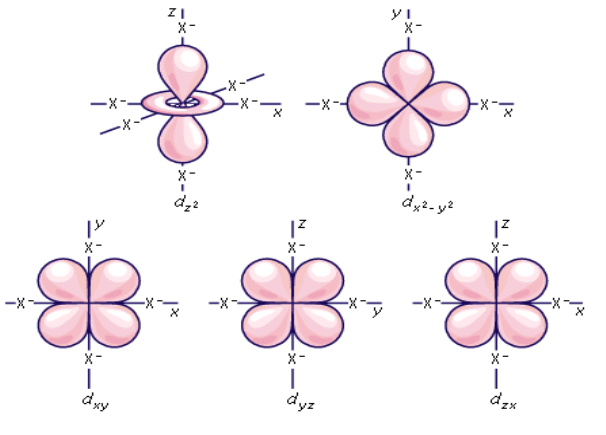
Shape of d orbital:
The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). Hence, we can say that there are five d-orbitals. These orbitals are designated as dxy, dyz, dxz, dx2–y 2 and dz2. Out of these five d orbitals, shapes of the first four d-orbitals are similar to each other, which is different from the dz2 orbital whereas the energy of all five d orbitals is the same.
The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called as the electronic configuration. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Electronic configuration describe the specific location of each electrons in atoms or molecules.
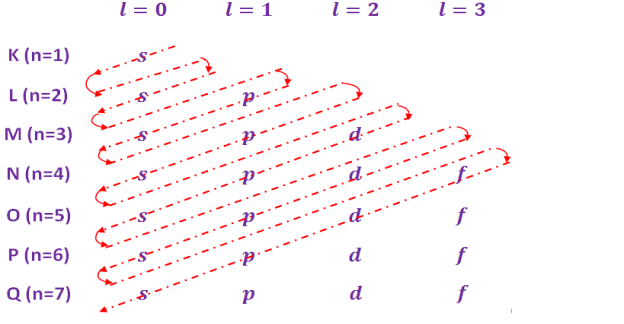
The electron distribution over various energy level is governed by the following rules –
The maximum number of electrons in any main energy level (shell) is given by, ‘2n2’, where, n is an integer and representing the “principal quantum number”. For different main energy levels the value of ‘n’ and maximum number of electrons are given in table below-
Sl. No. | Energy level or Orbit (shell) | Principal quantum number ‘n’ | Maximum Number of electrons (2n2) |
1 | K | 1 | 2×12 = 2 |
2 | L | 2 | 2×22 = 8 |
3 | M | 3 | 2×32 = 18 |
4 | N | 4 | 2×42 = 32 |
The each main shell (energy level) is subdivided into sub shells. These sub shell are called orbitals. These sub shells /orbitals are designated by s, p, d, f etc. with corresponding orbital quantum number, l = 0, 1, 2, 3, 4,…..(n-1) etc. The number of sub shells in any main shell is equal to “principal quantum number” ‘n’.
Atomic radii
Atomic radius is the radius of spherical atoms. Nonbonding atoms have a larger, more undefined radius, so when atomic radius is discussed as a periodic trend, what's usually meant is bonding atomic radius. These are the radius of atoms that are chemically bonded to one another. So, if the bond between two Cl atoms in Cl2 is 1.99 angstroms, we report chlorine's bonding atomic radius as about 0.99 angstroms. Further these values to estimate bond lengths between different elements in molecules.
Ionic Radii
Ionic radii are the radii of ions of elements. These distances are based on distances between ions in ionic compounds. Cations, or positively charged ions, are smaller than their "parent" atoms. This is because cations are formed when those outermost orbitals are vacated of electrons. This also decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many occupied orbitals and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely.
Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation.
H(g)→H+(g)+e−(1)(1)H(g)→H+(g)+e−
This energy is usually expressed in kJ/mol.
When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom.
Periodic Table and Trend of Ionization Energies
Ionization energies are dependent upon the atomic radius. Since going from right to left on the periodic table, the atomic radius increases, and the ionization energy increases from left to right in the periods and up the groups. Exceptions to this trend are observed for alkaline earth metals (group 2) and nitrogen group elements (group 15). Typically, group 2 elements have ionization energy greater than group 13 elements and group 15 elements have greater ionization energy than group 16 elements. Groups 2 and 15 have completely and half-filled electronic configuration respectively, thus, it requires more energy to remove an electron from completely filled orbitals than incompletely filled orbitals.
Alkali metals (IA group) have small ionization energies, especially when compared to halogens group. In addition to the radius, the number of electrons between the nucleus and the electron in the outermost shell has an effect on the ionization energy as well. This effect, where the full positive charge of the nucleus is not felt by outer electrons due to the negative charges of inner electrons partially cancelling out the positive charge, is called shielding. The more electrons shielding the outer electron shell from the nucleus, the less energy required to expel an electron from said atom. The higher the shielding effect the lower the ionization energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy.
Electron affinity:
Electron affinity is defined as the amount of energy released on the addition of electron in neutral atom to form an anion. The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favorable the electron addition process is. Not all elements forms stable negative ions in which case the electron affinity is zero or even positive.
Electron affinity increases going left to right across a period. The overall trend across a period occurs because of increased nuclear attraction.
Going down the group the electron affinity should decrease since the electron is being added increasingly further away from the atom. Less tightly bound and therefore closer in energy to a free electron.
Electron affinity=1/Atomic Size
1. Atomic size: If the atomic size is small, then there will be greater electron gain enthalpy because the effective nuclear forces will be greater in the smaller atoms and the electrons will be held firmly.
2. Nuclear charge: The greater the nuclear charge more will be the value for electron gain enthalpy because an increase in nuclear charge will increase the effective nuclear force on valence electrons.
Electro-negativity:
It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. Electro negativity can be measured on several scales. The most commonly used scale was designed by Linus Pauling. According to this scale, fluorine is the most electronegative element with a value of 4.0 and cesium is the least electronegative element with a value of 0.7.
Periodic Trends of electro negativity: On moving across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electro negativity increases across a period in the modern periodic table.
There is an increase in the atomic number as we move down the group in the modern periodic table. The nuclear charge also increases but the effect of the increase in nuclear charge is overcome by the addition of one shell. Hence, the value of electro negativity decreases as we move down the group. It is a general observation that metals show a lower value of electro negativity as compared to the non-metals.
Polarizability is a measurement of a tendency of electron cloud is distortion by an electric field. Typically the electron cloud will belong to an atom or molecule or ion. The electric field could be caused, E.g. By an electrode or a nearby cation or anion.
If an electron cloud is easy to distort, we say that the species it belongs to is polarizable.
Polarizability, which is represented by the Greek letter alpha, α, is experimentally measured as the ratio of induced dipole moment p to the electric field E that induces it:
α = p/E
The units of α are C m2 V-1.
Some elements in the periodic table have only one oxidation number or two oxidation numbers. But some have lot of oxidation numbers. Oxidation number of element in a compound can be positive or negative or may be zero.
In sodium compounds, sodium only forms +1 oxidation number.
But some types of atoms such as chlorine form various oxidation numbers like -1, 0, +1, +3, +5, +7 oxidation numbers in compounds.
Rules for the determination of Oxidation States:
- The oxidation state of an uncombined element is zero. This applies regardless of the structure of the element: Xe, Cl2, and large structures of carbon or silicon each have an oxidation state of zero.
- The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
- The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion.
- The more electronegative element in a substance is assigned a negative oxidation state. The less electronegative element is assigned a positive oxidation state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
Atomic Number | Element | Oxidation Number |
1 | Hydrogen | -1, 0, +1 |
2 | Helium | 0 |
3 | Lithium | +1 |
4 | Beryllium | +2 |
5 | Boron | +3 |
6 | Carbon | -4, -3, -2, -1, 0, +1, +2, +3, +4 |
7 | Nitrogen | -5, -4, -3, -2, -1, 0, +1, +2, +3 |
8 | Fluorine | -1, 0 |
10 | Neon | 0 |
The Coordination number of an atom in a molecule or a crystal refers to the total number of atoms, ion, or molecules bonded to the atom in question. ‘Ligancy’ is another term used to refer to the coordination number of an atom.
The atoms, ions, molecules that are bonded to the central atom are called ligands. The ligancy of molecules is calculated differently when compared to calculating the coordination number of a central atom in a crystal.
There exist multiple possible geometric combinations for each value of the coordination number for the central atom. These possible geometric shapes are tabulated below.
Coordination Number | Geometric Structure |
2 | Linear |
3 | T-shaped, Trigonal Pyramidal |
4 | Tetrahedral |
5 | Trigonal bipyramidal |
6 | Hexagonal Planar |
7 | Pentagonal bipyramidal |
8 | Hexagonal bipyramidal |
9 | Three face centered trigonal prism |
10 | Bi-capped square ant prism structure |
Geometries
On attraction of positively charged metal cation and the negative charge on the non-bonding electrons of the ligand the interaction between a transition metal and ligands arises.
As a ligand approaches the metal ion, the electrons from the ligand will be closer to some of the d-orbitals and farther away from others, causing a loss of degeneracy. The electrons in the d-orbitals and those in the ligand repel each other due to repulsion between like charges. Thus the d-electrons closer to the ligands will have a higher energy than those further away which results in the d-orbitals splitting in energy.
The splitting can be affected by following factors:-
• Metal ion nature
• Metals oxidation state.
• Ligand arrangement around the metal ion
• Metal coordination number
Spectrochemical Series: The ability of ligands to cause a large splitting of the energy between the orbital is essentially independent of the metal ion and the spectrochemical series is a list of ligands ranked in order of their ability to cause large orbital separations.
I- < Br- < SCN- ~Cl- < F- < OH- ~ ONO- < C2O42- < H2O< NCS- < EDTA4-<NH3 ~ pyr ~ en < bipy < phen < CN- ~ CO
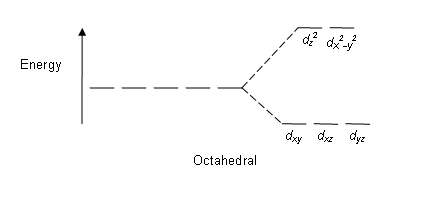
Octahedral: The crystal field stabilization energy (CFSE) is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d orbitals are split in a ligand field, some of them become lower in energy than before. For example, in the case of an octahedron, the t2g set becomes lower in energy. As a result, if there are any electrons occupying these orbitals, the metal ion is more stable in the ligand field by the amount known as the CFSE. Conversely, the eg orbitals are higher in energy. So, putting electrons in them reduces the amount of CFSE.
Square Planar: Crystal field stabilization is applicable to the transition-metal complexes of all geometries. The reason that many d8 complexes are square-planar is the very large amount of crystal field stabilization that this geometry produces with this number of electrons.
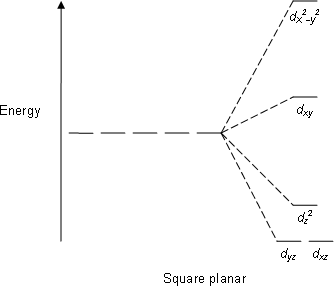
E.g; XeF4, PtCl2−
Tetrahedral:
dxy dxz dyz
The electron density (i.e., the lobes of the orbitals) lies nearest to the point charges.
dx2-y2 dz2
The electron density lies further away from the point charges.
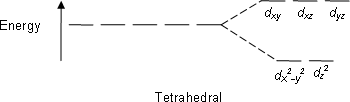
E.g; CH4
Principle of Pearson’s concept:
Pearson suggested a simple rule for predicting the stability of complexes formed between hard and soft acids and bases. “Hard acids prefer to bind (co-ordinate) with hard bases and soft acids prefer to bind with soft bases and gives stable complex compound”. It should be noted that the statement given above is not a theory or an explanation but it is simple rule of thumb which enables us to predict the relative stabilities of acid-bases adducts qualitatively.
Hard Acids and Bases
Hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom.
Examples of Hard Acids: H+, Li+, K+, Ca2+, Al3+, Sn4+, BF3, BCl3, CO2, RCO+, SO3, RMgX, VO2+, AlCl3
Hard bases are highly electronegative and of low polarizability.
Examples of Hard Bases: F-, OH-, NH3, N2H4, ROH, H2O, SO42-, PO43-
Hard bases react more readily to form stable compounds and complexes with hard acids.
Soft Acids and Bases
Soft acids consist of large low charge cations and molecules with relatively high energy occupied molecular orbitals. Soft acids are readily polarizable.
Examples of Soft Acids: Cs+, Cu+, Au+, Pt2+, Hg+, BH3, Br2, I2, RO+, quinones
Hard bases have low electronegative and low polarizability.
Examples of Soft Bases: H-, R-, CO, PR3, C6H6, SCN-
Soft bases react more readily and form stable compounds and complexes with soft acids.
Characteristics of HSAB:
Hard acid soft acid:
Hard Acid | Soft Acid |
Small iconic radius | Large iconic radius |
High positive charge | Low positive charge |
Low electro negativity | Intermediate electro negativity |
High energy LUMO | Low energy LUMO |
Hard base soft base:
Hard Base | Soft Base |
Small radius | Large radius |
High electro negativity | Intermediate electro negativity |
Weak Polarazibility | High Polarazibility |
High energy HOMO | Low energy HOMO |
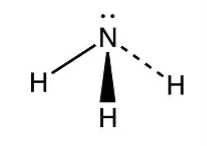
NH3:
Total valence electron = 5 + 3*1
= 8
=8/2
= 4
So,
The Hybridization = SP3
Bond pair =3
So,
Lone pair = 4–3 = 1
So,
Shape is pyramidal
H3O+:
H3O + is pyramidal in shape.
In H3O+, the central atom (oxygen) has three bond pairs and one lone pair and hence, it is sp3 hybridized.
You must know that ideal geometry for a sp3 hybridized central atom molecule is Tetrahedral. So the molecule should have Tetrahedral geometry in ideal condition ( a condition of all electron pairs on central atom being bond pairs only ).

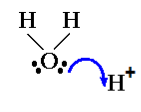
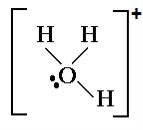
But this is not an ideal case. Here, three electron pairs are bond pairs while one electron pair is lone pair creating distortion in molecule leading to a pyramidal shape of H3O+.
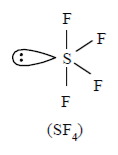
SF4:
With the context of VSEPR theory, electrons of the molecule can be counted to determine the electron geometry
Sulfur: 6 valence electrons
Fluorine: 7x4 valence electrons
Total: 34 valence electrons
The sulfur can be kept in the middle because fluorine tends to make single bonds. Therefore, we can keep 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available.
As a result, we have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, we get a seesaw molecular geometry.
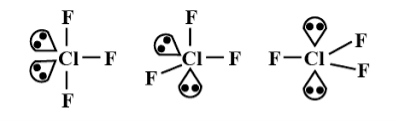
CIF3:
Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). These are arranged in a trigonal bipyramidal shape with a 175° F (axial)-Cl-F (axial) bond angle.
The two lone pairs take equatorial positions because they demand more space than the bonds. The result is a T-shaped molecule.
Postulate of Kossel of ionic bonding:
- In the periodic table, the highly electronegative halogens and the highly electropositive alkali metals are separated by the noble gases. Therefore one or small number of electrons are easily gained and transferred to attain the stable noble gas configuration.
2. The formation of a negative ion from a halogen atom and a positive ion from an alkali metal atom is associated with the gain and loss of an electron by the respective atoms.
3. The negative and positive ions so formed attain stable noble gas electronic configurations. The noble gases have filled outer shell electronic configuration of eight electrons with a general representation ns2 np6.
4. The negative and positive ions are bonded and stabilized by force of electrostatic attraction.
E.g.
(i) Na ( [Ne] 3s1) -- loss of e --- > Na+ + e ( [Ne])
Where,
[Ne] = electronic configuration of Neon
(ii) Cl + e ([Ne]3s2 3p5 ) - gain of e-- > Cl-1([Ar])
Where,
[Ar] = electronic configuration of Argon
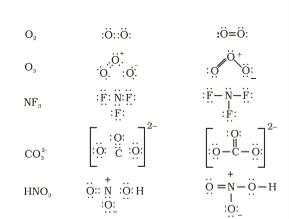
- The total number of electrons required for writing the structures is obtained by adding the valence electrons of the combining atoms. For example, in the CH4 molecule there are eight valence electrons available for bonding i.e.; 4 from carbon and 4 from the four hydrogen atoms.
- The negative charge means addition of one electron in anions while the positive charge would result in subtraction of one electron from the total number of valence electrons in cation. For example, for the CO32– ion, the two negative charges indicate that there are two additional electrons than those provided by the neutral atoms. For NH4+ ion, one positive charge indicates the loss of one electron from the group of neutral atoms.
- Knowing the chemical symbols of the combining atoms and having knowledge of the skeletal structure of the compound, it is easy to distribute the total number of electrons as bonding shared pairs between the atoms in proportion to the total bonds.
- In general the least electronegative atom occupies the central position in the molecule. For example in the NF3 and CO32–, nitrogen and carbon are the central atoms whereas fluorine and oxygen occupy the terminal positions.
- After accounting for the shared pairs of electrons for single bonds, the remaining electron pairs are either utilized for multiple bonding or remain as the lone pairs. The basic requirement being that each bonded atom gets an octet of electrons.
Reference Books:
1. Engineering Chemistry by Jain and Jain, Dhanpat Rai Publishing Co.
2. Engineering Chemistry Willey India Publisher
3. Engineering Chemistry by Marry Jane & Shultz, Cencage Learning Publisher
4. Engineering Chemistry by N. Krishnamurthy, P. Vallinaygam and D. Madhavan,
Prentice Hall of India Pvt. Ltd.
5. Engineering Chemistry by K. Sesha Maheswaramma and Mridula Chugh, Pearson India Education Pvt Ltd.
6. Engineering Chemistry by B K. Sharma, Krishna Prakashan Media (P) Ltd.
7. A textbook of Engineering Chemistry by Shashi Chawla, Dhanpatrai Publishing Co. Ltd.
8. Fundamentals of Biotechnology by B D Singh, Kalyani Publisher. New Delhi.
9. Essential of Physical Chemistry by Bahl and Tuli., S Chand & Co. Ltd, New Delhi.
10. Introduction to Nano Science by N N. Lindsay, Oxford University Press.
11. NANO: The Essentials by T Pradeep Tata McGraw-Hill Publishing Company, New Delhi.