Unit III
Metals, Alloys and Corrosion
Content
The combinations of metallic elements, such as iron, titanium, aluminum, and gold, which may also contain small amounts of non-metallic elements, such as carbon, nitrogen, and oxygen are metallic materials. Metals are mixed with other elements to form an alloy instead of using pure. This is usually necessary to obtain the required properties of the material. The factors that effect the choice of metals and alloys as biomaterials are:
(i) physical and mechanical properties
(ii) degradation of the material
(iii) biocompatibility
State: Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (Gallium is liquid on hot days).
Luster: The ability to reflect the light of a metal surface is called as luster.
Malleability: Metals have the ability to withstand hammering and can be made into thin sheets known as foils. For example, a sugar cube sized chunk of gold can be pounded into a thin sheet that will cover a football field.
Ductility: Metals can be drawn into wires. For example, 100 g of silver can be drawn into a thin wire about 200 meters long.
Hardness: All metals are hard except sodium and potassium, which are soft and can be cut with a knife.
Valency: Metals typically have 1 to 3 electrons in the outermost shell of their atoms.
Conduction: Metals are good conductors because they have free electrons. Silver and copper are the two best conductors of heat and electricity. Lead is the poorest conductor of heat. Bismuth, mercury and iron are also poor conductors
Density: The mass of every single volume of a metal is the metal density.
Melting and Boiling Points: Metals have high melting and boiling points.
It is the mixture of metallic solid solution that composed of two or more elements. E.g.: Brass, pewter, phosphor bronze, steel. Alloys typically refer to metals that are formed from the mixture of two or more elements. One of those elements must be a metallic element, but other constituents may not always be metallic. Alloys give single solid phase microstructure. The partial solutions give two or more than those phases that may or may not be the homogenous in distribution.
The classification of alloys on the basis of their composition is:
1) Ferrous Alloys
2) Non Ferrous Alloys
Ferrous Alloys: Ferrous alloys are metals that consist mostly of iron that is Fe. Steel is an iron-based alloy containing typically less than 1% carbon, where iron frequently contains 2% or more carbon. They are produced in larger quantities than any other metallic material. Their mechanical properties can be improved by heat treating and, in the case of steels, by working. Stainless steels were developed to resist corrosion and generally contain 12% or more chromium, and may contain nickel in any amount up to or even exceeding the chromium content based upon the mechanical properties desired and application.

Non Ferrous Alloys: A metal is defined as non-ferrous it means that it does not have a significant amount of iron in its chemical composition. That means nearly all metal alloys have some trace, or non-significant, amount of iron in their composition. This does not make them ferrous alloys though. Non-ferrous alloys generally have iron compositions of less than one percent as measured by weight. If iron constitutes a large percentage of a metal, such as if it is the first or second most abundant element in the metal’s chemical composition, then the metal is considered ferrous.
Plain carbon steels are iron-carbon alloys in which the properties are primarily derived from the presence of carbon. Some incidental elements like manganese, silicon, sulphur and phosphorus are present in small amounts due to the method of making steels and, not to modify the mechanical properties.
Alloy steels are those steels when, one, or more of the alloying elements are intentionally added to plain carbon steels to enhance, or induce some property, or properties. It is a bit difficult to make a clear cut distinction between plain carbon and alloy steel.
However, AISI (American Iron and Steel Institute) adopted the following definition. ‘Carbon steels are regarded as steels-containing not more than 1.65% manganese, 0.60% silicon and 0.60% copper, all other steels being regarded as alloy steels. Common alloying elements are nickel, chromium, vanadium, silicon, manganese, etc.
Stainless steels
Stainless steel is a family of alloy steels usually containing 10 to 30% of chromium. In conjunction with low carbon content, chromium imparts remarkable resistance to corrosion and heat. Other elements such as nickel, molybdenum, titanium, aluminium, niobium, copper, nitrogen, phosphorus or selenium, may be added to increase corrosion resistance to specific environments, enhance oxidation resistance, and impart special characteristics.
Properties of Stainless Steel:
- Corrosion resistant.
- High tensile strength.
- Very durable.
- Temperature resistant.
- Easy formability and fabrication.
- Low-maintenance
- Attractive appearance.
- Environmentally friendly
Cu Alloy
Copper alloys are those metal alloys that have copper as their principal component. They possess high resistivity against corrosion. The best known traditional types are bronze; where tin is a significant addition, and brass, using zinc instead. Both of these are imprecise terms, having both been commonly referred to as lattens in the past.
Applications:
- Power transmission lines
- Architectural applications
- Cooking utensils
- Spark plugs
- Electrical wiring, cables and busbars
- High conductivity wires
- Electrodes
- Heat exchangers and refrigeration tubing
- Plumbing
Alnico:
Alnico is the name for an iron alloy that primarily consists of iron, aluminium, nickel & cobalt. Alnico alloys have ferromagnetic properties which makes it strong permanent magnets. These magnets also show excellent stability in a wide range temperature. There are effective in temperatures upto 1000∘F.
Composition of alnico is
1. Al (Aluminium)
2. Ni (Nickel)
3. Co (Cobalt)
sometimes it also includes titanium
Duralium:
Duralium is a metal consist of an alloy of aluminium, copper, magnesium and manganese. Duralium is a special kind of metal, it is hard made by subjecting it to heat treatment. It may be well spun, tempered, riveted, welded or machinated. The duralumin, which is effectively given heat treatment, can be effectively being resistant to corrosion. It can carry heavy loads, and is ductile. It is specially suited for aircraft construction.
Composition of duralumin
1. Cu (Copper)
2. Mg (Magnesium)
3. Mn (Manganese)
Corrosion is the electrochemical process that occurs in various forms such as chemical forms and atmospheric forms. On the contact of acid sucstance with iron it pretend to form rust. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture. When steel is exposed to water, the iron particles are lost to the water’s acidic electrolytes. The iron particles then become oxidized, which results in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and flow through the steel to another area of the steel known as the cathodic area.
Mechanism of Corrosion:
(I) Dry or Chemical Corrosion-
The reaction of metal with water vapour or gas at high temperature causes the metal to corrode chemically. This is the redox process in which the electron of the metal are passed directly to the substance in the environment. The metal corrodes generally in the metal which is in higher contact with water.
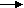 3Fe + 4H2O Fe3O4 + 4H2
3Fe + 4H2O Fe3O4 + 4H2
 3Fe + 2O2 Fe3O4
3Fe + 2O2 Fe3O4
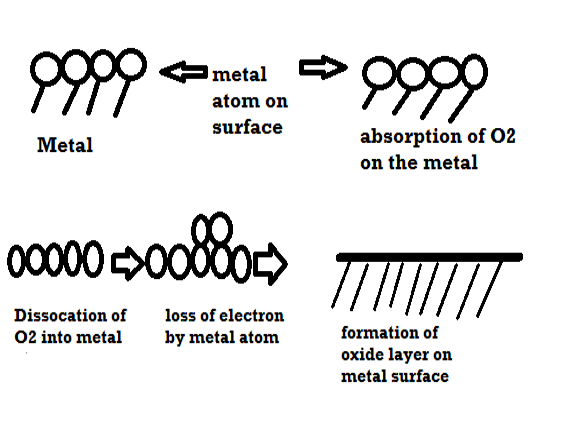
i) Due to oxygen
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
(II)Wet or Electrochemical corrosion- Mechanism
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by teh elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.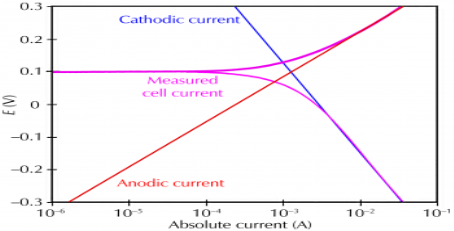
Evolution of hydrogen type: Evolution of Hydrogen Electrochemical corrosion with evolution of H2 occurs in acidic Environment At anode
Fe →Fe2+ + 2e-(oxidation /corrosion)
At cathode
2H+ + + 2e-→H2
Overall reaction Fe +2H+ →Fe
Explanation (1) Iron tank which acts as an anode undergoes corrosion as Fe atoms from the tank pass into the acidic solution as Fe++ ions as shown in reaction above. Since nothing but loss of Fe atom as Fe++ ions i.e. corrosion. (2) Free electrons accumulate at cathode Hydrogen ions present in acidic solution take up these electrons forming H2 gas as shown in the reaction above. H2 gas liberates in the form of bubbles near the cathode. Thus, hydrogen evolution type of corrosion is nothing but displacement of H+ from acidic solution by metal ions. All the metals above H2 in the electro chemical series get dissolved in acidic solution with simultaneous evolution of hydrogen. In hydrogen evolution type of corrosion anode h as a large area (like metallic tank) and cathode has a smaller area.
Absorption of oxygen:Absorption of Oxygen If electrolyte is neutral or alkaline aqueous solution, corrosion takes place by absorption of O2 rusting of iron in water containing dissolved oxygen occurs by oxygen absorption mechanism. At anodic area iron will dissolve by oxidation. The surface of iron is usually coated with the thin film of iron oxide. But if this iron oxide film develops some cracks, anodic areas are created on the surface ; while the metal act as cathodes. Here the anodic areas are small surface while rest of the surface of the metal forms large cathodes.
At anode,
Fe →Fe2+→2e-
The electrons flow to cathodic area through and will be accepted by O2.
At Cathode
Fe2+ +2OH→Fe (OH)2
If enough O2 is present, ferrous hydroxide easily oxidizes to ferric hydroxide 2Fe (OH) 2 + ½ O2 +H2O→2Fe(OH)3
Ferric hydroxide rust This product called yellow rust, which is nothing but Fe2O3.H2O If O2 is limited, the corrosion product will be black unhydrous magnetite, Fe3O4. If environment is aqueous solution of NaCl containing dissolved O2 NaCl →H2O→Na+ + Cl-
At cathode
Na+ + OH→NaOH
At anode
Fe2+ + 2Cl→FeCl2
Ferrous Chloride Both the products NaOH, FeCl2 are soluble in water they react with each other and ferrous hydroxide precipitates which further oxidizes to ferric hydroxide Fe(OH)3. 4 Fe (OH)2 + 2H2O + O2 →4Fe(OH)3 -Fe2O3. H2O yellow rust.
Types of Corrosion-
Galvanic cell corrosion:Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
Concentration cell corrosion (differential aeration principle):The uneven supply of oxygen to the same metal component leads to the formation of oxygen concentration cells that is called as the differential aeration theory of corrosion. It is the type of electrochemical corrosion that affects the metals such as steel and iron. The less oxygenated part behaves anodic while the more oxygenated part cathodic. Since cathodic reactions involve consumption of oxygen, the more oxygenated part behaves cathodic and less oxygenated pan behaves anodic. The reaction occurs because oppositely charged electrons flow between the smaller anode and larger cathode. Positively charged cations meeting negatively charged anions forming corrosion product and a resulting pit in the metal, otherwise known as pitting corrosion. In a gutter, pipe, tank or similar the anode is just below the waterline. This is where the oxidation occurs, corrosion product forms and a pit develops weakening the metal.
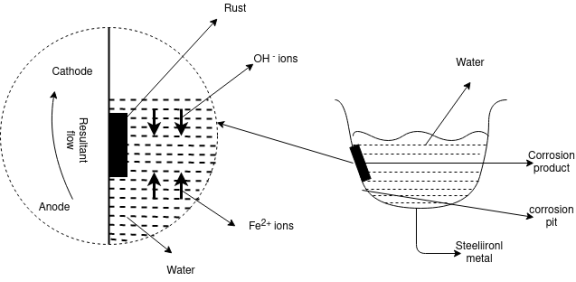
Pitting corrosion: This type of corrosion occurs at certain conditions, there is a accelerated corrosion in some areas rather than the uniform corrosion over the substance. This condition includes low level of concentration of oxygen or high concentration of chlorides.
Intergranular corrosion:Intergranular corrosion is strongly associated with the properties and microstructure of a metal. A well-known example of IGC is the sensitisation of austenitic 18Cr—8Ni stainless steels. In the temperature range of 538–927°C, insoluble chromium carbides, Cr23C6, precipitate at the grain boundaries. This precipitate is a product of the reaction between chromium and the carbon diffusing along the grain boundaries. Below 538°C, the carbon remains relatively immobile, and above 927°C the chromium carbides are soluble. Formation of chromium-rich precipitates quickly depletes chromium from a region immediately adjacent to the grain boundary. This reduces the alloy’s ability to generate chromium oxide protective films and leads to an increased susceptibility for localized attack in the grain boundary region.
Welding is a common source of localized heating that can sensitize a material. IGC is often observed within the heat affected zone after welding a material susceptible to heat sensitisation.
Stress corrosion:Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments. The chemical environment that causes SCC for a given alloy is often one which is only mildly corrosive to the metal. Hence, metal parts with severe SCC can appear bright and shiny, while being filled with microscopic cracks. This factor makes it common for SCC to go undetected prior to failure. SCC often progresses rapidly, and is more common among alloys than pure metals. The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.
Factors affecting the rate of corrosion-
(i)Nature of metal:
Position of metal in galvanic series.
If position is higher in galvanic series then it carrode faster
While for 2 metal the difference between them shows the corrosion ratio.
(ii)Nature of corroding environment: Iron and steels are the most versatile, least expensive and most widely used materials for the construction of many engineering systems. They are unequaled in their range of mechanical and physical properties with which they can be endowed by alloying and heat treatment. Their main disadvantage is that they have poor corrosion resistance in even relatively mild environments unlike stainless steels. Corrosion can reduce the load-carrying capacity of a component either by reducing its size or by pitting. Pitting not only reduces the effective cross section in the pitted region but also introduce stress raisers, which initiate cracks. Any technique that reduces or eliminates corrosion will extend the life of a component and increase its reliability. In addition to corrosion prevention methods, it is important to design and select materials for improving the overall corrosion performance of components. The three interrelated factors that drive the selection of materials for corrosion control are the corrosivity of the environment, the corrosion resistance of the material and the acceptable rate of attack.
Corrosion failures during service are likely if suitable materials with appropriate coatings are not selected for the fabrication of components of critical systems. Corrosion significantly affects the efficiency of systems. Failures can be minimized or reduced significantly by controlling corrosion. The possible methods of controlling corrosion are the application of coatings, linings, metal cladding and corrosion inhibitors, and alloying. Among these, the application of suitable coatings appears to be a promising method of corrosion control in terms of cost effectiveness and service life. DMR-1700 is a recently developed ultrahigh-strength low-alloy steel with improved mechanical properties compared with other aerospace-grade low-alloy steels. However, its corrosion characteristics have not been investigated in detail, although some corrosion studies have been carried out.
In the present investigation, the effect of the environment on the corrosion characteristics of DMR-1700 steel is studied in detail, the properties of the steel under various environmental conditions are compared and the degradation mechanisms involved are considered. On the basis of the results of the investigation, we recommend the use of the material with a suitable protective coating for the fabrication of components for various applications. In the study, the surface morphologies of the corroded steels in different environments were observed using a scanning electron microscope (SEM) to determine the nature of corrosion. We also successfully developed a high-performance coating for DMR-1700 steel to enhance its resistance against corrosion. The developed coating is discussed, and its application is recommended for effective protection against corrosion. The use of such an advanced material with a high-performance coating will help enhance the efficiency of systems by reducing failures during service.
Changing Mediums: Change in the environment provides a versatile means for reducing corrosion. Typical changes in the medium that are often employed are lowering temperature, decreasing velocity, removing oxygen or oxidizers, and changing concentration.
Lowering temperature: This usually causes a pronounced decrease in corrosion rate. However, under some conditions, temperature changes have little effect on corrosion rate. In other cases, increasing temperature decreases attack. This phenomenon occurs as hot, fresh or salt water is raised to the boiling point and is the result of the decrease in oxygen solubility with temperature. Boiling sea water is therefore less corrosive than hot sea water.
Decreasing velocity: Velocity generally increases corrosive attack, although there are some important exceptions. Metals and alloys that passivate, such as stainless steels, generally have better resistance to flowing mediums than stagnant solutions. Very high velocities should be always avoided where possible, because of erosion-corrosion effects.
Changing concentration: Decreasing corrosive concentration is usually elective. In many processes, the presence of a corrosive is accidental. E.g.: corrosion by the water coolant in nuclear reactors is reduced by eliminating chloride ions. Many acids such as sulphuric and phosphoric are virtually inert at high concentrations at moderate temperatures.
A corrosion inhibitor is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.
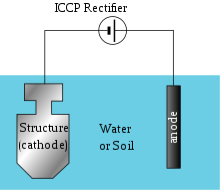
This is the technique used to control the corrosion on the surface of metal by formation of cathode layer on an electrochemical cell. There are 2 types of cathodic protections:
(i) Sacrificial Anodic Protection
(ii) Impressed Current Cathodic Protection
Sacrificial Anodic Protection:
The metal surface can be protected from the corrosion by connecting it wire to a more anodic metal. The sacrifice of this more anodic metal to save the metal form corrosion is called as the Sacrificial Anode. The most common metal used for this purpose are Mg, Zn, Al etc.
Applications:
(i) The underground cable and pipeline protection from soil erosion.
(ii) Ships and boat protection from marine corrosion.
(iii) Prevention of rusty water by inserting Mg sheets or rods into domestic water boiler or tanks.
Impressed Current Cathodic Protection:
This is the type of corrosion protection which consist of sacrificial anodes that is connected to an external power source. The external power source is DC power supply, that provides the sufficient current to drive electrochemical reaction required for the cathodic protection to occur.
Reference Books:
1. Engineering Chemistry by Jain and Jain, Dhanpat Rai Publishing Co.
2. Engineering Chemistry Willey India Publisher
3. Engineering Chemistry by Marry Jane & Shultz, Cencage Learning Publisher
4. Engineering Chemistry by N. Krishnamurthy, P. Vallinaygam and D. Madhavan,
Prentice Hall of India Pvt. Ltd.
5. Engineering Chemistry by K. Sesha Maheswaramma and Mridula Chugh, Pearson India Education Pvt Ltd.
6. Engineering Chemistry by B K. Sharma, Krishna Prakashan Media (P) Ltd.
7. A textbook of Engineering Chemistry by Shashi Chawla, Dhanpatrai Publishing Co. Ltd.
8. Fundamentals of Biotechnology by B D Singh, Kalyani Publisher. New Delhi.
9. Essential of Physical Chemistry by Bahl and Tuli., S Chand & Co. Ltd, New Delhi.
10. Introduction to Nano Science by N N. Lindsay, Oxford University Press.
11. NANO: The Essentials by T Pradeep Tata McGraw-Hill Publishing Company, New Delhi.