Unit IV
Polymers and Fibers
Content
The process in which the simpler molecules combine together to form very large molecule having high molecular weight is known as polymerization. The molecule is known as polymer. The different ways of doing polymerization are –
By opening a double bond
Ex:
NCH2 = CH2 (CH2 – CH2)n
Ethane polythene
NCH2=CH (CH2 – CH)n
Cl Cl
Vinyl chloride polyvinyl chloride
Types of polymerization:
Polymers can be synthesized by the following polymerization processes:
- Addition polymerization or chain polymerization
- Condensation or step or step growth polymerization
III. Copolymerisation
Addition polymerization:
The addition polymerization is the process in which the liniking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerisation and the polymer is an exact multiple of the original monomeric molecule.
Condensation polymerization:
An intermolecular reaction involving two different bifunctional reactants with affinity for each other and taking place through repeated condensation reaction is known as condensation polymerization.
Copolymerisation:
Addition polymerisation involving a mixture of two or more suitable or compatible monomers gives a copolymer and the process is known as copolymerization. A reaction in which a mixture of two or more monomers is allowed to undergo polymerisation is known as copolymerization. The polymer is known as copolymer.
E.g.: Copolymerisation of styrene and methyl methacrylate
A polymer which can be converted from harmless gaseous products by action of biological enzyme and water, is called as biodegradable polymers 3 components are important in the biodegradation of organic polymers.
A) Organisms: - like pseudomonas, bacillus, protozoa, acetobacter, various fungi,etc.
B) Environment: - suitable temp, moist condition, presence of salts, suitable ph.
C) Nature of polymer: -
- Polymer chain should contain bond which can be hydrolysed or oxidized by the enzyme action o, s.
- High aromatic they are tough for degradation
Hydrophilic chain backbone of polymer contains O,N,3. Atoms easily degrade.
3. Hydrophilic end groups
4. More amorphous nature of polymer
5. Small size of polymer material
Example: - PHBV
It is a co – polymer of hydroxy butyric acid and 3 hydroxyvaleric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.
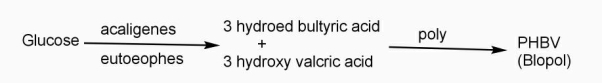
STRUTURE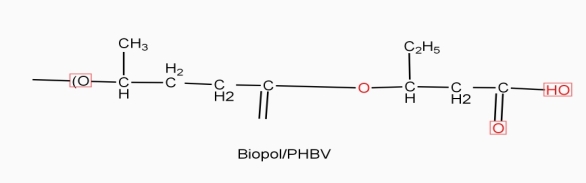
Properties: -
- It is highly crystalline, soluble in chloroform
- It has melting point 180° c
It is thermo softening polymer, soft, flexible.
Free Radical Polymerization: The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called as the free radical polymerization. In order to obtain the wide variety of different polymers and material composites the free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction make it one of the most versatile forms of polymerization which allows a facile reactions of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.
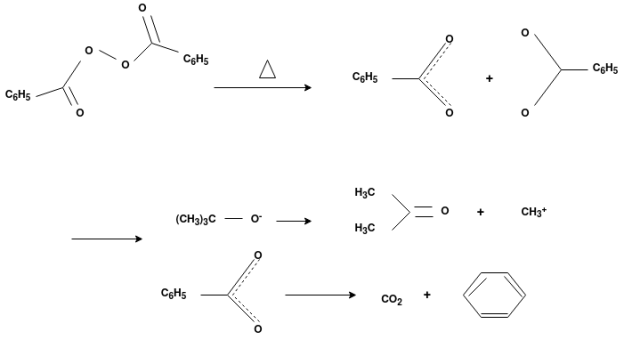
Radical Initiators
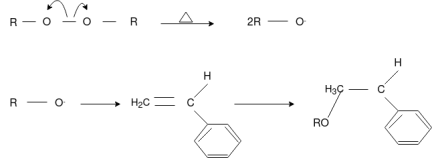
Chain Initiation
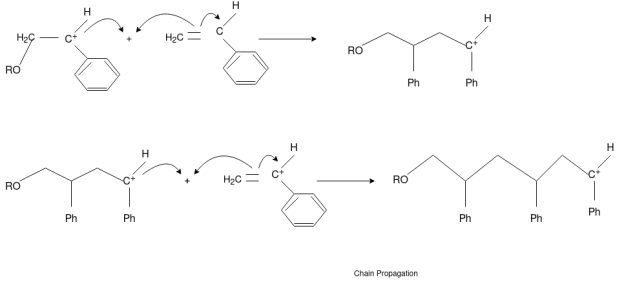
Chain Propagation
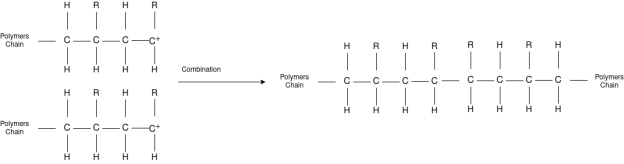
Chain Termination
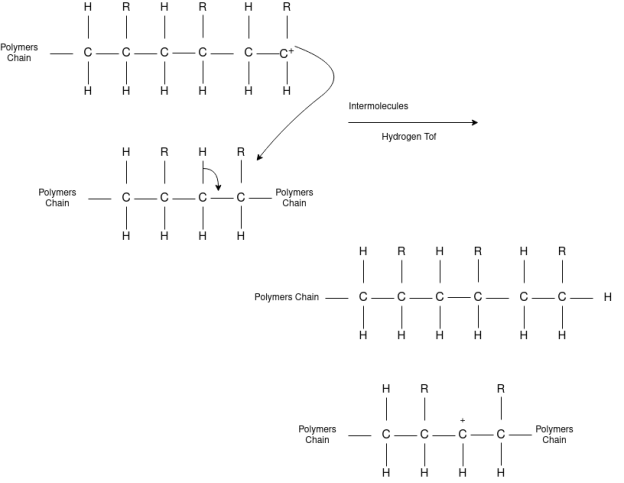
Chain Transfer Reaction
Ionic Polymerization: Alkenes can polymerize under the influence of both cationic and anionic initiators. As with free radical polymerizations, the ionic processes involve initiation, propagation and (sometimes) termination steps.
Cationic Polymerization: Cationic polymerizations are typically initiated by carbocations, generated by protonation of an alkene with a strong acid such as sulfuric or tri-fluoro methane sulfonic acid.
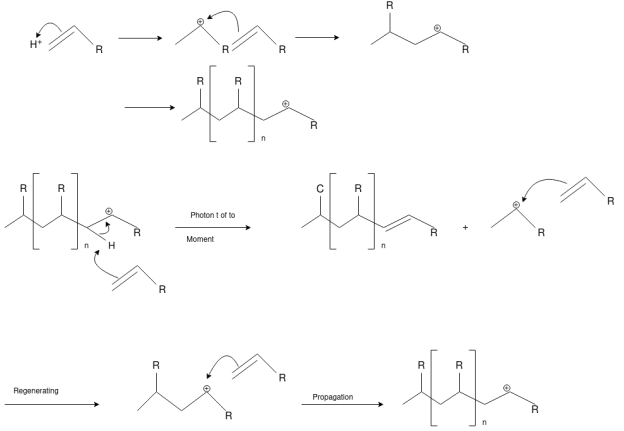
Anionic Polymerization: Anionic polymerizations are typically initiated by carbanions such as organolithium compounds.
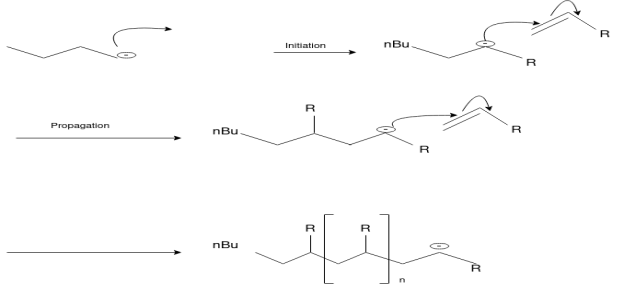
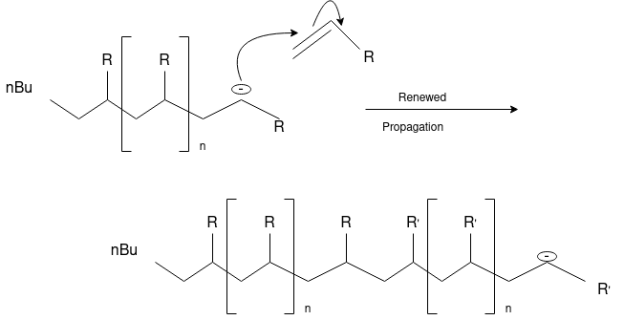
Rubbers also known as elastomers, they are high polymers, which have elastic properties in excess of 300%.
Natural Rubber:- Natural Rubber is a high molecular weight hydrocarbon polymer represented by the formula (C5H8)x. It is obtained from a milk emulsion called latex by tapping the bark of the tree. “Heve a brasiliensis”. It is a polymer of isoprene units.
n H2C = C – CH = CH2 ( H2C – C = C – CH2 )n
CH3 CH3
The polymer chain of natural rubber is made of 2000 to 3000 monomer units.
Processing of Natural Rubber:- By cutting the bark of rubber tree the milky colloidal rubber milk is obtained. The main constituent of rubber latex is 25-45% of rubber and the remaining are water, protein & resinous materials. The rubber latex is coagulated by using 5% acetic acid and made in to sheets. The rubber sheets are cured under mild heat and then subjected to further processing.
Crepe rubber:- To the rubber latex a small amount of sodium bisulphate is added to bleach the colour and feed in to roller which produce 1mm or more thickness sheets which are dried in air at about 40- 500C. The dried thin sheet of rubber are known as “smoked crepe rubber”.
Mastication:- Rubber becomes soft and gummy mass when subjected to severe mechanical agitation. This process is known as mastication. Mastication followed by the addition of certain chemical (compounding) which is carried out on roll mills or internal mixers. After mastication is complete, the rubber mix is prepared for vulcanization.
Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers. The most common vulcanization agent is sulfur. It forms bridges between individual polymer molecules when heated with rubber. Often a catalyst and initiator is added to accelerate the vulcanization process. The cross-linked elastomers have much improved mechanical properties. In fact, unvulcanized rubber has poor mechanical properties and is not very durable.
- Mixing of crude rubber with about 5-30% of sulfur (cross-linking agent) and other additives such as:
- Activator (commonly zinc oxide or stearic acid),
- Accelerator (guanidines, thiazoles, dithiocarbamates, xanthates, thiurams) ,
- Coagulants (acetic acid, calcium chloride),
- Anti-oxidants (amines, phenolics, phosphites),
- Color pigments,
- Surfacants,
- Softeners,
- Ant-foaming agents,
- Anti-tack agents (Rosin derivates, coumarone-indene resins, aliphatic petroleum resins, alkyl-modified phenol-formaldehyde resins).
Slow cross-linking starts at this stage. It is neccessary to avoid active vulcanization during mixing, which may cause cracks formation at the molding stage.
- Molding (shaping) the rubber mixture. The rubber must be shaped prior to heating stage since cross-linking makes shaping impossible.
- Heating the mixture to 250-400ºF (120-200ºC). Increased temperature speeds up the vulcanization process resulting in fast and complete cross-linking. C-S bonds replace C-H bonds linking chain polyisoprene molecules. Each link is formed by one to seven sulfur atoms.
Applications:
(i) The major application of natural rubber is in the manufacture of tyres.
(ii) In heavy duty tyres, the major portion of the rubber used is natural rubber.
(iii) The tank linings in chemical plants where corrosive chemicals are stored are prepared from rubber.
(iv) To reduce machine vibrations, rubber is used for sandwiching between two metal surfaces.
(v) Foam rubber is used for making cushions’, matrices, padding etc. toys and sports items are manufactured from natural rubber.
Biodegradation: -
It is a processes of converting polymer material into harmless simple gaseous products by the action on enzymes of microorganism and water.
A polymer which can be converted from harmless gaseous products by action of biological enzyme and water, is called as biodegradable polymers 3 components are important in the biodegradation of organic polymers.
A) Organisms: - like pseudomonas, bacillus, protozoa, acetobacter, various fungi, etc.
B) Environment: - suitable temp, moist condition, presence of salts, suitable ph.
C) Nature of polymer: -
- Polymer chain should contain bond which can be hydrolysed or oxidized by the enzyme action o, s.
- High aromatic they are tough for degradation
Hydrophilic chain backbone of polymer contains O,N,3. Atoms easily degrade.
3. Hydrophilic end groups
4. More amorphous nature of polymer
5. Small size of polymer material
Example: - PHBV
It is a co – polymer of hydroxy butyric acid and 3 hydroxyvaleric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.
STRUTURE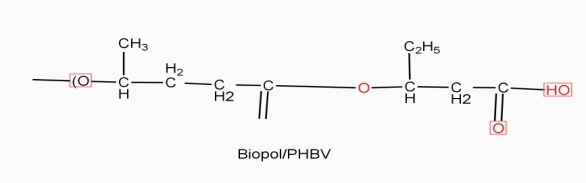
Properties: -
- It is highly crystalline, soluble in chloroform
- It has melting point 180° c
- It is thermo softening polymer, soft, flexible.
Limitations: -
- Such polymers are very costly
- They cannot manufacture on a large scale
- Don’t possess high mechanical strength
Applications: -
- Used for moulded articles
- Used for films for packing and lamination
- It is used in medical field – for organs transplants, surgical, and orthopaedic, operations.
- It is used in agriculture field – for manufacturing fertilizers and pesticides.
The polymers have ability to conduct electricity are called as conducting polymers.
Structural requirements of polymer: -
- Polymer containing conjugation double bonds system
- Polymer with the higher crystallinity and high planarity
- Presence of aromatic rings in a polymer chain.
- Polymer has a linear structure.
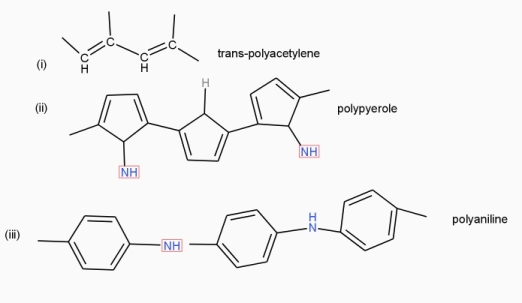
Poly acetylene: -
Structure: -
- The polymer consists of along chain carbon atoms with alternating single and double bonds between them. Each carbon possesses one hydrogen atom.it is obtained by Ziegler matter ayteslys.
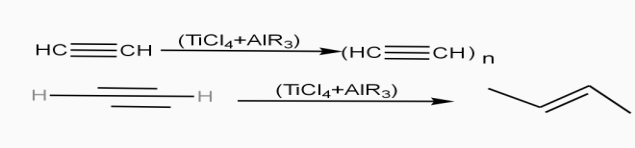
- Doping of polymer: - addition of impurities
- Intrinsic polymers: - the polymer which conduct electricity on their own is called as intrinsic polymers.
- Extrinsic polymers: - the polymers which conduct electricity by doping processes are called as extinct polymers.
In doping process, polymer is either oxidized or reduced.
There are two type of doping
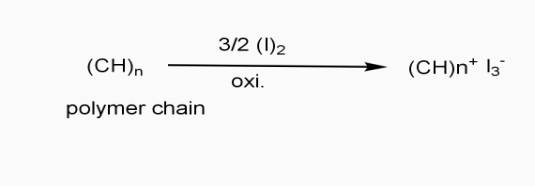
a] oxidative / p type doping
- Includes ICPS with Lewis acid.
- Oxidizing reagents are used (e.g. Iodine vapours)
- Oxidation taking place
- +VE change develops on polymer chain.
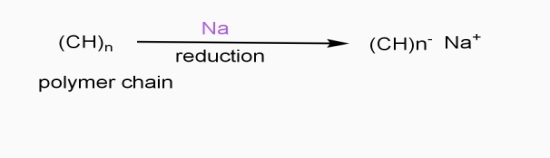
b] Reductive / n type doping
- Includes ICPS with Lewis bases
- Reducing reagents are used (e.g.Na,Li,etc.)
- Reduction taking place
- - ve charge develops on polymer chain
Applications: -
- For making rechargeable light weight batteries.
- In electronic devices, transistors, diodes.
- In optical display device.
- In solar cells.
- In telecommunication system.
PMMA :-
Preparation:
This is an important thermoplastic resin. It is obtained by polymerization of methyl methacrylate which is an ester of methyl acrylic acid, CH2=C(CH3)COOH, in presence of acetyl peroxide or hydrogen peroxide. It is an acrylic polymer.
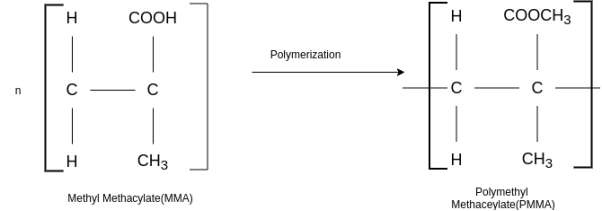
Properties:
(i) It is hard, fairly rigid material with a high softening point of about 130-14rfC.
(ii) It becomes rubber like at a temperature above 6oC.
(iii) It has outstanding shape-forming properties due to wide span of temperature from its rigid state to viscous.
(iv) It has high optical transparency.
(v) It has high resistance to sunlight and ability of transmission light accurately.
Uses:
They are used for making lenses, optical parts of instruments, air craft, light fixture, artificial eyes, wind screen, bone splints, decorative articles etc. PMMA is found in paint and used in window glasses.
Polyethylene:
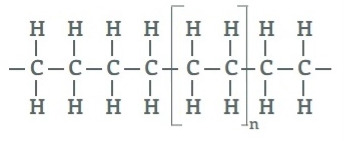
Polyethylene is a lightweight, durable thermoplastic with variable crystalline structure. It is one of the most widely produced plastics in the world. Polyethylene is used in applications ranging for films, tubes, plastic parts, laminates, etc. in several markets (packaging, automotive, electrical, etc.).
Polyethylene is made from the polymerization of ethylene or ethene monomer. Polyethylene chemical formula is (C2H4)n.
Uses:
- Polyethylene is used in packaging products. This plastic is often employed for the production of plastic bags, plastic films, bottles, etc.
- Polyethylene is used in crates, trays, jugs that carry milk or fruit juices.
- High-density polyethylene is used in toys, garbage containers and ice trays.
- HDPE is also used in ropes, fishing nets, agricultural nets, and industrial fabrics.
- Low-density polyethylene (LDPE) is widely used in the production of squeeze bottles, garbage bags, laminations, and food packaging due to its high flexibility and low cost.
- LDPE is also used in pipes and fittings.
- Polyethylene is also used for cable jacketing since it is a good insulator of electric current.
Polypropylene
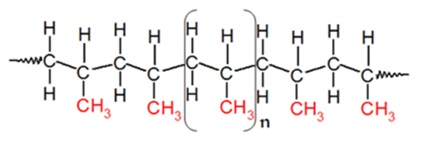
Polypropylene is a tough, rigid and crystalline thermoplastic produced from propene or propylene monomer. It is a linear hydrocarbon resin. The chemical formula of polypropylene is (C3H6)n. PP is among the cheapest plastics available today.
Uses
1) Polypropylene is used as packing material due to its high resistance and lower cost.
2) Polypropylene is used in several household products like as furniture, luggage, toys etc.
3) It is used in automobiles. E.g.: fender liners, battery cases, tray, bumpers, instrumental panels, door trims etc.
PVC
The monomer used for the manufacture of PVC is vinyl chloride.Vinyl chloride is prepared by treating acetylene with HCl at 60-800 c and in presence of a metal oxide catalyst.
Metal oxide
CH CH + HCl CH2 = CHCl
(Acetylene) 60 – 800 c Vinyl chloride
Poly vinyl chloride is produced by heating vinyl chloride in presence of benzyl peroxide or H2O2.
Benzoyl peroxide
n CH2 = CH ( CH2 – CH )n Cl Polymerisation Cl
Vinylchloride at 30 – 800 c PVC
Uses:
- PVC is used in making seals & gaskets, which have to withstand high temperature.
- It is also used for insulation of electrical items and for making non-sticky surface coating, particularly for cooking utensils.
- Teflon used as insulating material for motors, transformers, cables, wires, fitting etc.
Natural Rubber:- Natural Rubber is a high molecular weight hydrocarbon polymer represented by the formula (C5H8)x. It is obtained from a milk emulsion called latex by tapping the bark of the tree. “Heve a brasiliensis”. It is a polymer of isoprene units.
n H2C = C – CH = CH2 ( H2C – C = C – CH2 )n
CH3 CH3
The polymer chain of natural rubber is made of 2000 to 3000 monomer units.
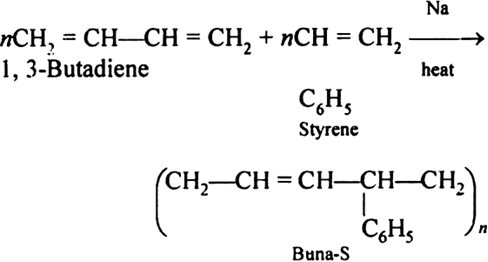
Synthetic rubbers (Buna-S):
Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrine. Buna-S is the replacement of natural rubber while styrene, 2 monomers and butadiene play a major role in its derivation where as these 2 monomers is polymerized by two basically different process i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called as cold rubber. The obtained rubber is called as the Styrene Butadiene Rubber (SBR).
Physical property of fibers:
1) Mechanical Properties: The mechanical property includes important parameters like breaking strength, breaking elongation, elastic recovery and toughness. One of the most-performed tests on textile fibres, yarns and fabrics is the tensile stress-strain test.
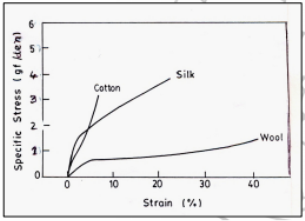
Stress strain curves of natural fibers
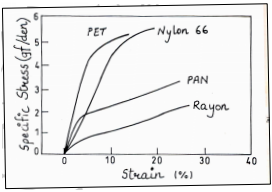
Stress strain curves of man-made fibers
2) Moisture absorption: It become very uneasy if the clothes do not absorb perspiration and moisture in hot and humid weather while if the cloth absorb then it creates a equilibrium with outside atmosphere to reduced discomfort. The natural fibres absorb moisture better than man-made fibres.
Percentage moisture Re gain = Mass of absorbed water in specimen * 100
Mass of dry specimen
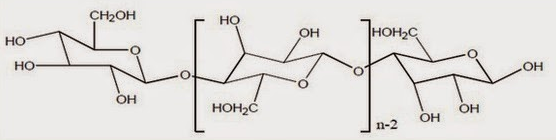
Viscose Rayon is also called as the artificial silk as it was generated from the cellulosic fibre. Rayon is made by using cuprammonium process and is called as cupra rayon. It is a man-made fibre that composed of 100% regenerated cellulose. It is made up of cotton linters or wood pulp that is obtained from the spruce and pine trees.
Viscose rayon is one of the most absorbent of all textiles. It is more absorbent than cotton or linen and is exceeded in absorbency only by wool and silk. A variation of rayon is classified as high wet modulus (HWM) rayon or polynosic rayon. This type of rayon is launderable.
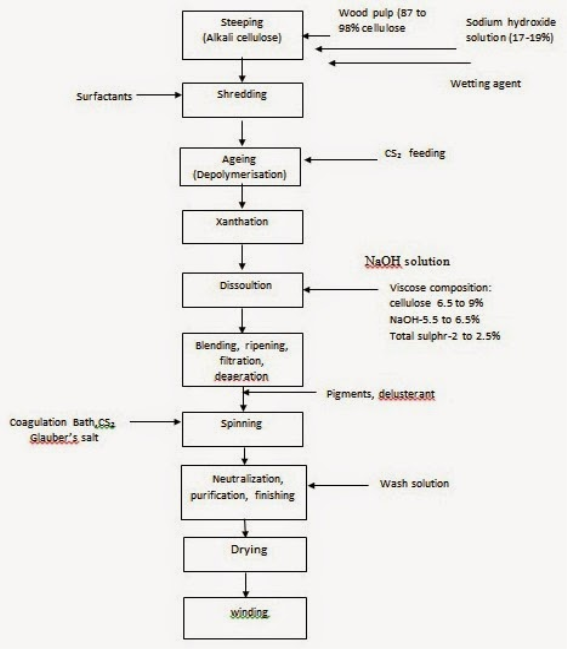
Manufacturing Process of Viscose Rayon:
Physical properties of viscose rayon:
1) Tensile strength is greater than that of the wool.
2) Viscose rayon has greater elasticity than cotton or linen but less than wool or silk.
3) Viscose rayon is good conductor of heat.
4) Viscose rayon is a one of the most absorbent of all textiles.
5) Viscose rayon fabrics tend to shrink more than cotton fabrics of similar construction.
Nylon-6:
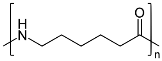
Nylon 6 is also called as the Polycaprolactam which is developed by Paul Schlack. They are semicrystalline polyamide.They have smooth surface and are featureless as a glass rods. Nylon 6 can be modified using comonomers or stabilizers during polymerization to introduce new chain end or functional groups, which changes the reactivity and chemical properties. It's often done to change its dyeability or flame retardance. Nylon 6 is synthesized by ring-opening polymerization of caprolactam. When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4–5 hours, the ring breaks and undergoes polymerization. Then the molten mass is passed through spinnerets to form fibres of nylon 6.
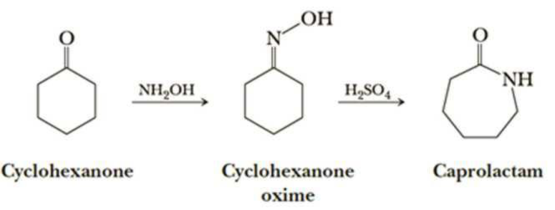
On further heating of Caprolactam at 533K with inert atmosphere of nitrogen for about 4-5 hours it gives
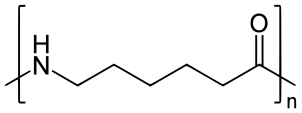
(Nylon-6)
Application:
(i) Nylon 6 is widely used for gears, fittings, bearings and automotive industry for various parts and as a material for power tools and housings.
(ii) It is used as threads in bristles for toothbrushes, surgical sutures and strings for acoustic and classical instruments.
(iii) It is used in production of large variety of ropes, filaments, nets as well as knitted garments.
(iv) It can also be used in gun frames, such as those used by Glock, which are made with composite of Nylon 6 and other polymers.
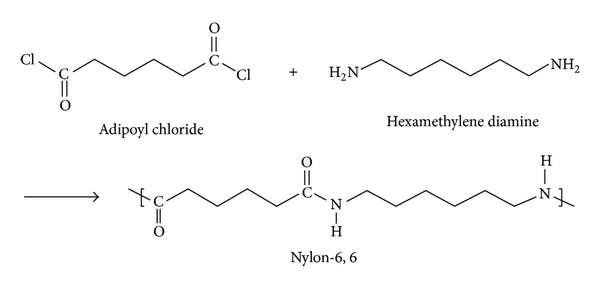
Nylon 6,6:
Nylon 6,6 is a polyamide made from adipic acid and hexamethylenediamine by polycondensation. The resulting polymer is extruded into a wide range of fiber types. The fiber are drawn and stretch in a process that increases their length and reorients the material molecules parallel to one another to produce a strong elastic filament. The thermo plasticity of nylon permits permanent cripping of the fibres and provides bulk and stretch properties. The Nylon 6,6 is prepared from Nylon salt i.e., prepared by reacting the hexamethylene diamine and adipic acid in boiling methanol. The comparatively insoluble salt precipitates out from methanol. A 60% of aqueous solution of salt is then run into a stainless steel autoclave together with a trace of acetic acid to limit the molecular weight. The vessel is sealed and purged with oxygen free nitrogen and the temperature raised to 220oC at about the pressure of 1-7 MPa is developed. After 1-2 hours the temperature is raised to 270-280oC and steam blend off to maintain the pressure.
Liquid Crystals: There are three state of matter i.e.; Solid, Liquid, Gas. Liquid Crystal is the fourth state that enters under the right conditions and consequences. The matter which shows the property between the conventional Liquid and Solid Crystals. This can be simplified in this manner that the Liquid Crystals may flow like the liquid but the molecule of the crystal may be in the crystal form. The molecules in the liquid crystal do not exhibit any positional order instead they represent the orientational order. They tend to orient in one direction more than other hence this direction is known as the director of the Liquid Crystals.
There are several phases in Liquid Crystal.
1-Mesomorphic Phases
1.1-Thermotropic Liquid Crystal
1.2-Lyotropic Liquid Crystal
Thermotropic Liquid Crystal class can be prepared by heating. The known crystals are organic compounds. Ex.- bis-(p-methylbenzal)-p.
While at another end the Lyotropic Liquid Crystal are prepared by the mixture of two or more components.
Applications
Liquid Crystal Display:
Polarization: Light wave is an electromagnetic wave in which time barring electric field vector E bar and magnetic field vector V bar are mutually perpendicular to each other and perpendicular to the direction of propagation of light wave. In a light wave electric field vector vibration occurs in different planes such light wave are unpolarised light wave. If it is confined in particular direction then such light wave is polarized light wave. This phenomenon is called Polarization. When unpolarised light pass through the polarizer then the vibration of polarizer tend to polarize the light. While in Liquid Crystal Display the intensity of light can be controlled at a given point by changing the orientation of molecules. The optical properties of the liquid crystal can often are manipulated by subjecting it to a magnetic or electric field that changes the orientation of the molecules. The number of regions or the pixels in there per unit area responsible for the following conditions:
Very low Resolution
Low Resolution
Improved Resolution
High Resolution
Working of Liquid Crystal Display:
It is based on change in optical properties of LCD which is caused by electric fields. In LCD the thin film of Liquid Crystal usually the Nematic crystal is sandwich in between thin transparent electrodes. The arrangement is provided to apply an electric field across a small area of this thin film usually called as pixel. This is used to apply electric field across a small area of this thin film of liquid crystal. TFT Matrix means thin film transistor matrix. This is the arrangement which provide electric field to the small region of this liquid crystal film on both side of transparent electrode polarized filter is placed. One polarizing filter placed between backlight and the LC films while another one is placed between liquid crystal film and screen. The white light is meant to pass through the filter then after that this light fall on Liquid Crystal film the liquid crystal changes the polarization of light and this change is controlled by applying electric field to various point on the Liquid Crystal film. This electric field is applied at various point which are called as Pixels with help of TFT matrix, change in polarization lead to change in intensity of light at different points. The combine effect of change in intensity at all the point form the image which can be seen at the screen. Eg.Computer Screen, Watches etc.
Reference Books:
1. Engineering Chemistry by Jain and Jain, Dhanpat Rai Publishing Co.
2. Engineering Chemistry Willey India Publisher
3. Engineering Chemistry by Marry Jane & Shultz, Cencage Learning Publisher
4. Engineering Chemistry by N. Krishnamurthy, P. Vallinaygam and D. Madhavan,
Prentice Hall of India Pvt. Ltd.
5. Engineering Chemistry by K. Sesha Maheswaramma and Mridula Chugh, Pearson India Education Pvt Ltd.
6. Engineering Chemistry by B K. Sharma, Krishna Prakashan Media (P) Ltd.
7. A textbook of Engineering Chemistry by Shashi Chawla, Dhanpatrai Publishing Co. Ltd.
8. Fundamentals of Biotechnology by B D Singh, Kalyani Publisher. New Delhi.
9. Essential of Physical Chemistry by Bahl and Tuli., S Chand & Co. Ltd, New Delhi.
10. Introduction to Nano Science by N N. Lindsay, Oxford University Press.
11. NANO: The Essentials by T Pradeep Tata McGraw-Hill Publishing Company, New Delhi.