Unit VI
Fuel and Combustion
Content
Fuels :-
A fuel is the substance which on combustion produces a large amount of heart.
Classification of chemical fuels :-
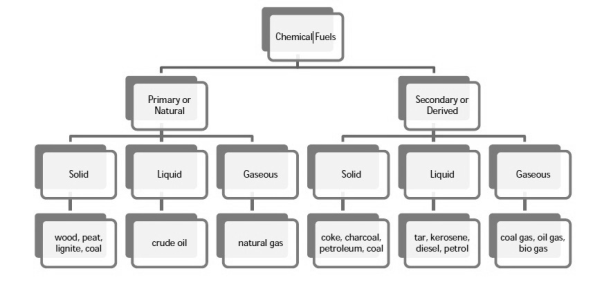
Chemical fuels are classified on the basis of occurrence into :-
- Primary ( natural )
- Secondary ( derived ) types / manmade .
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter .( if the liquid is volatile , then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction :- a bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . The lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its centre.
- One of these electrodes is provided with a ring to hold the crucible containing fuel .there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter :-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter , which can record the rise in temperature of welter due to absorb in a heat generated .
- There are insulator stands between calorimeter and water jacket.
Accessories :-
- There is a pellet press to convert the powder of solid fuel to pellet form .for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
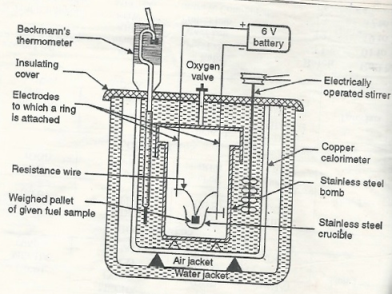
Fig: Bomb Caloriemeter
Working :-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible .keep the crucible in the ring of the electrode . Keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs .keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
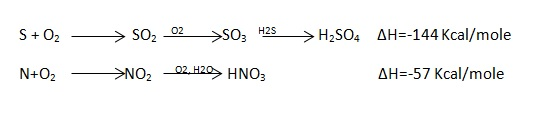
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5 .these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached .after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. Are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Calculations :-
Gross calorific value of fuel = l calories / gm
Rise in temperature of water = ( t2 – t1 )
Heat liberated by burning fuel = heat absorbed by water and calorimeter
Therefore ,
XL = ( W+W) ( T2 – T1 )
G.CV = L = 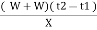 cal / gm
cal / gm
Note :-
Water equivalent of calorimeter – set ( w ) is determined by burning fuel of known gross calorific value and using the above equation.
Standard gross calorific values of some pure fuels are,
Naphthalene = 9622 Cal / gm
Benzoic acid = 6325 Cal / gm
Camphor = 9292 Cal / gm
Salicylic acid = 5269 Cal /gm
NCV for the fuel is calculated as below .
If H is percentage of hydrogen in the fuel, then the heat taken by water formed during combustion to convert it into steam is = 0.09h * 587 Cal / gm
And
NCV = GCV – 0.09h * 587 Cal / gm or kcal / kg.
Corrections :-
To get more accurate results , the following corrections should be considered.
- Fuse wire correction ( F )
- Acid correction ( a )
- Cooling correction ( tc )
- Fuse wire correction ( f ) :-
Out of the total obtained little heat is given out by fuse wire when the current is passed for s – 10 sec .to start the combustion. Hence it must be subtracted. Are exothermic and the heat measured includes a small share by these acid formations.
2. Cooling correction ( te ) :-
If the time taken for water in calorimeter to cool from maximum temperature attained to the room. Temperature is t minutes and average cooling rate is dt / min , then the cooling correction to be added to rise in temperature is t.dt .
- The actual rise in temperature would have been ( t23 – t1 ) had the heat liberated been absorbed by water and calorimeter rapidly and there would have no loss of heat from the set by radiation.
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
Boy’s Calorimeter:
Principle :-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
- Gas burner
- Combustion chamber ( chimney )
- Thermometers
- Insulating cover
- Gas burner :-
- There is a gas burner in which a known volume of gas is burnt at a known pressure .the gas is burnt at the rate of 3 – 4 lit. Per minute.
3. Combustion chamber ( chimney ) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of its water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometers to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere .there are three holes for exhaust gas, water inlet and condensed steam.
Working :-
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady ( temperature in and around the combustion chamber ) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
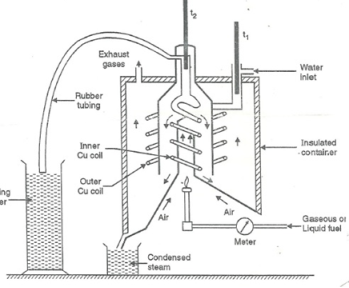
Fig: Boy’s gas calorimeter
Calculations :-
- First convert the volume of gas burnt to volume of gas at STP .let this STP volume be V
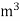 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 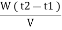 Kcal /
Kcal / 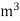
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
=  *587 kcal /
*587 kcal / 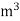
Therefore ,
NCV = GCV -  *587
*587
NCV = 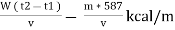
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 The fuel should be easily available.
2 It should be dry and should have less moisture content.
3 Dry fuel increases its calorific value.
4 It should be cheap, easily transportable and has high calorific value.
5 It must have moderate ignition temperature and should leave less ash after combustion.
6 The combustion speed of a good fuel should be moderate.
7 It should not burn spontaneously to avoid fire hazards.
8 Its handling should be easy and should not give poisonous gases after combustion.
9 The combustion of a good fuel should not be explosive.
There are two methods to analyze coal: ultimate analysis and proximate analysis. The ultimate analysis determines all coal component elements, solid or gaseous and the proximate analysis determines only the fixed carbon, volatile matter and moisture and ash percentages. The ultimate analysis is determined in a properly equipped laboratory by a skilled chemist, while proximate analysis can be determined with a simple apparatus.
- Measurement of moisture: The determination of moisture content is carried out by placing a sample of powdered raw coal of size 200- micron size in an uncovered crucible, which is placed in the oven kept at 108 +2 °C along with the lid. Then the sample is cooled to room temperature and weighed again. The loss in weight represents moisture.
- Measurement of volatile matter: A fresh sample of crushed coal is weighed, placed in a covered crucible, and heated in a furnace at 900 + 15 oC. The sample is cooled and weighed. Loss of weight represents moisture and volatile matter. The remainder is coke (fixed carbon and ash).
- Measurement of carbon and ash: The cover from the crucible used in the last test is removed and the crucible is heated over the Bunsen burner until all the carbon is burned. The residue is weighed, which is the incombustible ash. The difference in weight from the previous weighing is the fixed carbon. In actual practice Fixed Carbon or FC derived by subtracting from 100 the value of moisture, volatile matter and ash.
- Proximate analysis: The proximate analysis indicates the percentage by weight of fixed carbon, volatiles, ash, and moisture content in coal. The amounts of fixed carbon and volatile combustible matter directly contribute to the heating value of coal. Fixed carbon acts as a main heat generator during burning. High volatile matter content indicates easy ignition of fuel. The ash content is important in the design of the furnace grate, combustion volume, pollution control equipment and ash handling systems of a furnace.
- Fixed carbon: Fixed carbon is the solid fuel left in the furnace after volatile matter is distilled off. It consists mostly of carbon but also contains some hydrogen, oxygen, sulphur and nitrogen not driven off with the gases. Fixed carbon gives a rough estimate of the heating value of coal.
LPG is a mixture of propane and butane with a small percentage of unsaturated Propylene and Butylenes and some lighter C2 as well as heavier C5 fractions. Also, propane (C3H8), Propylene (C3H6), normal and iso-butane (C4H10) and Butylene (C4H8) are included in the range of LPG. LPG may be defined as those hydrocarbons, which are gaseous at normal atmospheric pressure, but may be condensed to the liquid state at normal temperature, by the application of moderate pressures. Although they are normally used as gases, they are stored and transported as liquids under pressure for convenience and ease of handling. Liquid LPG evaporates to produce about 250 times volume of gas. LPG vapour is denser than air: butane is about twice as heavy as air and propane about one and half times as heavy as air. Consequently, the vapour may flow along the ground and into drains sinking to the lowest level of the surroundings and be ignited at a considerable distance from the source of leakage. In still air vapour will disperse slowly. Escape of even small quantities of the liquefied gas can give rise to large volumes of vapour / air mixture and thus cause considerable hazard. To aid in the detection of atmospheric leaks, all LPG’s are required to be odorized. There should be adequate ground level ventilation where LPG is stored. For this very reason LPG cylinders should not be stored in cellars or basements, which have no ventilation at ground level.
Methane is the main constituent of natural gas and accounting for about 95% of the total volume. Other components are: Ethane, Propane, Butane, Pentane, Nitrogen, Carbon Dioxide, and traces of other gases. Very small amounts of sulphur compounds are also present. Since methane is the largest component of natural gas, generally properties of methane are used when comparing the properties of natural gas to other fuels. Natural gas is a high calorific value fuel requiring no storage facilities. It mixes with air readily and does not produce smoke or soot. It did not contain sulphur. It is lighter than air and disperses into air easily in case of leak.
Biogas refers to a gas produced by the biological breakdown of organic matter in the absence of oxygen. Biogas refers to a mixture of gases produced by the anaerobic decomposition of organic matter such as agricultural waste, municipal waste, plant residue, food waste etc. Biogas consists of methane, carbon dioxide along with the small amount of hydrogen sulphide, and moisture. Biogas originates from biogenic material and is a type of bio fuel.
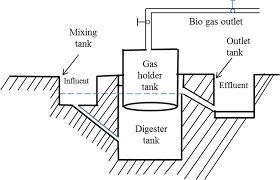
Biogas Plant:
The biogas plant made up of a dome-like structure. Organic material such as discarded food residue, fats, sludge, cow dung etc. are mixed with water and fed to the digester through the inlet. The digester is a sealed chamber where anaerobic decomposition of organic matter takes place. After a few days, the organic matter completely decomposes to generate gases like methane, carbon dioxide, hydrogen and hydrogen sulphide. These gases are then drawn through pipes from the storage tank above the digester and distributed through decentralization channels to nearby centers for use.
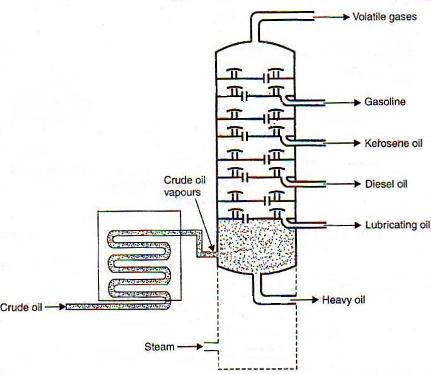
The separation of miscible liquids into its simpler forms is called as the fractional distillations. This separation begins when the mixture is heated at certain temperature where the fractions of the mixture start to vaporize. Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil and kerosene. The distillation process helps in separating these components effectively.
Crude oil is added in the chamber and is heated with high-pressure steam. The mixture starts boiling and vapor is formed. At this point, various substances enter into the vapor phase. The vapor rises up in the fractional distillation column which consists of several plates. The plates have holes that allow the vapor to pass through it. The temperature is usually kept low at the top of the fractionating column. Here, components with the highest boiling point will condense in the lower part of the column while substances with a low boiling point will condense at the top. The condensed vapors or liquid fractions are then removed from the sides of the column. The collected liquid fractions can further be passed through condensers to cool them even more.
Cetane number and octane number both are used to measure the tendency of the fuel ignition. The cetane number refers to the ease with which fuel ignites easily at a relatively low temperature. The Research Octane number and Motor Octane number used in determination of a single cylinder test engine in the laboratory while Road Octane Number measures the anti knock performance in an actual vehicle under road driving conditions. Cetane rating indicates the cold starting ability of diesel fuel that are mostly recommended by the automakers of about 45. High cetane rating means the fuel will ignite easily from heat and pressure and burn quickly.
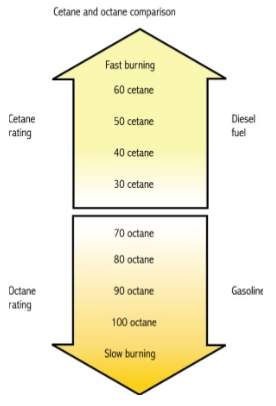
Cetane Rating
Fuel | Research Octane Number | Motor Octane Number | Cetane Number | Boiling Point (oC) |
Ethanol | 107 | 89 | 5 | 79 |
Methane (LPG) | 120 | 120 | 0 | -161.66 |
Diesel | -25 | - | 45-55 | 140-360 |
Gasoline | 92-98 | 80-90 | 0-5 | 37-205 |
Reference Books:
1. Engineering Chemistry by Jain and Jain, Dhanpat Rai Publishing Co.
2. Engineering Chemistry Willey India Publisher
3. Engineering Chemistry by Marry Jane & Shultz, Cencage Learning Publisher
4. Engineering Chemistry by N. Krishnamurthy, P. Vallinaygam and D. Madhavan,
Prentice Hall of India Pvt. Ltd.
5. Engineering Chemistry by K. Sesha Maheswaramma and Mridula Chugh, Pearson India Education Pvt Ltd.
6. Engineering Chemistry by B K. Sharma, Krishna Prakashan Media (P) Ltd.
7. A textbook of Engineering Chemistry by Shashi Chawla, Dhanpatrai Publishing Co. Ltd.
8. Fundamentals of Biotechnology by B D Singh, Kalyani Publisher. New Delhi.
9. Essential of Physical Chemistry by Bahl and Tuli., S Chand & Co. Ltd, New Delhi.
10. Introduction to Nano Science by N. Lindsay, Oxford University Press.
11. NANO: The Essentials by T Pradeep Tata McGraw-Hill Publishing Company, New Delhi.