Unit - 13
Engineering Materials
Content:
Material is nothing but a matter of substance used to create a certain thing. Engineering materials are the materials used to for the application of engineering works. Based on the mechanical, physical, chemical and manufacturing properties materials are selected according to the application.
Mechanical properties of the materials are strength (compressive or tensile), toughness, stiffness, elasticity, plasticity, ductility, brittleness and hardness.
Physical properties of materials are density, conductivity (thermal or electrical), acoustical (sound transmission or absorption), optical, combustibility.
Chemical properties of materials are composition (oxide or compound), acidity or alkalinity, weathering corrosion.
Manufacturing properties of materials are castability, machinability rating, machining speeds and feeds and for dimensioning purpose shape and size.
The selection of material for the required functioning application is based on some of the following factors:
- Stresses to which the work piece or component will be subjected.
- Corrosion resistance.
- Temperature, wear and tear resistance.
- Flexibility and rigidity.
- Easiness of the manufacturing process.
- Cost effectiveness for the product development.
- Availability of the material.
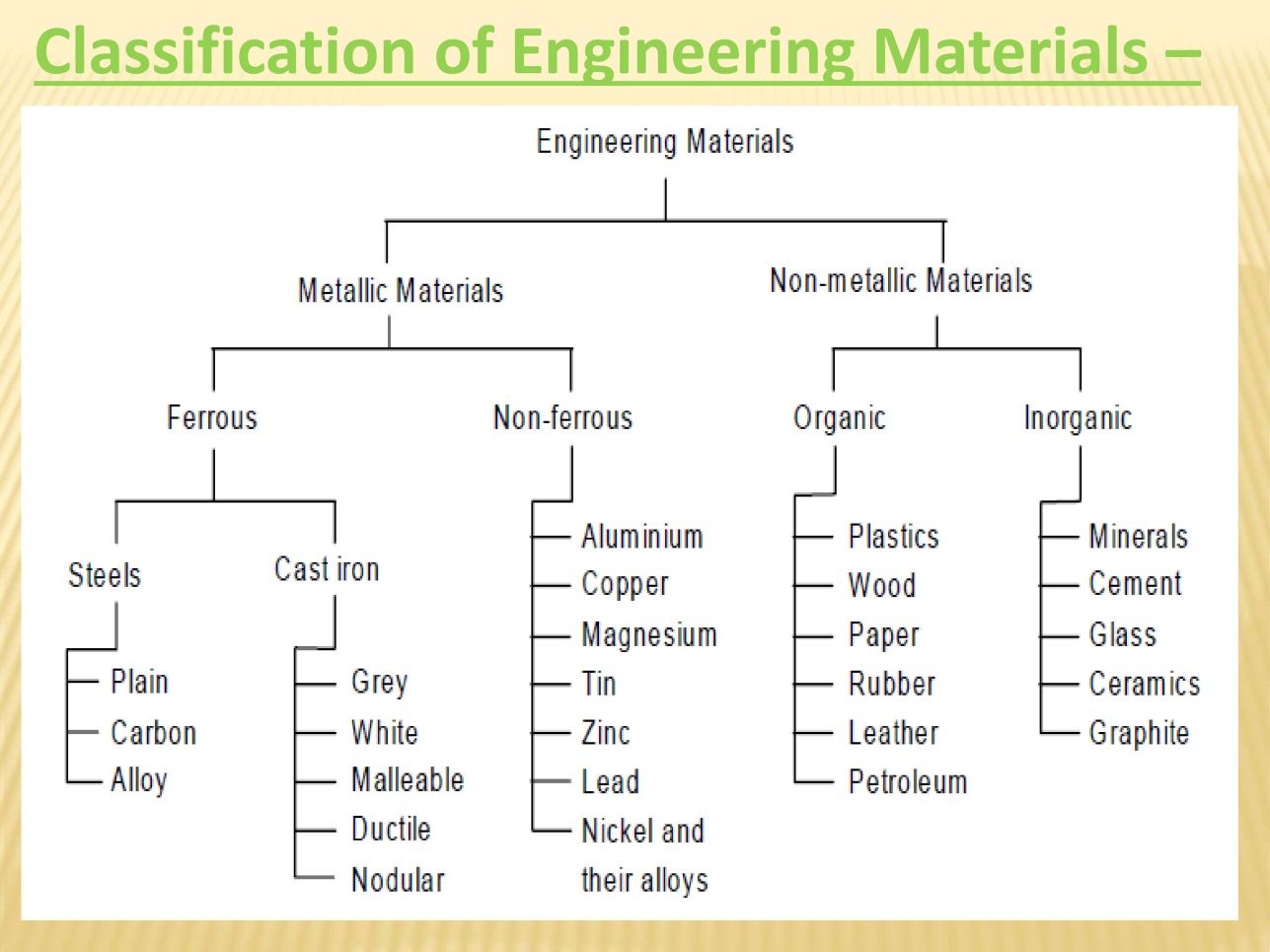
Classification of Engineering Material:
Depending upon the nature of substance materials are classified as
- Metals and alloys
- Ferrous metals
- Non-ferrous metals
- Non-metals
- Ceramics
- Polymers
- Composites
- Semi-conductors
- Bio materials
1. METALS AND ALLOYS:
Metallic materials are combination of metallic elements.
The most prominent property of metals is electrons are non-localized i.e. in atomic arrangement outer valence electrons do not belong to individual atom rather it belongs to whole bulk of material. Non-localized electron is free to carry charge to conduct electricity. Hence, they are good conductors for electrical and thermal charge.
Metals have lustrous appearance. At normal temperature majority of the materials are in solid state, but some metals like mercury lies in liquid state.
Based upon the presence of iron content metals are named as
- Ferrous metals
- Non-ferrous metals
Ferrous metals:
The primary content of ferrous metals is iron and carbon. Ferrous metals are magnetic and are vulnerable to rust when exposed to moisture. Wrought iron won’t rust due to purity and stainless steel due to presence of chromium.
E.g.: iron, steel, etc.
Due to their magnetic property ferrous metals are used in motor and electrical applications.
Non-Ferrous Metals:
Iron is not primary content. Due to the non-presence of iron these metals have high resistance to rust and corrosion and they are non-magnetic.
E.g.: copper, brass, aluminum, tungsten, lead, zinc, gold, etc.
Alloy:
Alloy is a combination of two or more metals. It is named based on metallic bonding character. It is of two types’ ferrous metal alloy and non-ferrous metal alloy. Cast iron is an alloy made from iron, carbon and silicon. Brass is an alloy of copper and zinc.
Applications:
- Due to their toughness and ability to sterilize at high temperatures metals are used as needles, surgical blades.
- Due to their strength and ability to withstand heavy weights metals like iron and steel are used in construction.
- Metals like gold, silver, platinum, etc. are used in jewellery.
- Metals are used in machines and automobiles as they can withstand high temperature, pressure and workloads.
- Aluminium and titanium play important role in light weight category for aircraft alloys.
- Tungsten is used in high temperature applications.
Timber is a type of wood which has been processed into beams and planks. It is also known as “lumber” in US and Canada. Basically, timber or Lumber is a wood or firewood of growing trees. Any wood capable of yielding a minimum dimensional size can be termed as a timber or lumber. It is a stage in the process of wood production. Timbers are used for the structural purpose. Those woods which are adapted for building purposes are timbers. Finished timber is supplied in standard sizes for the industry. Timber is used for building houses and making furniture.
Types of Timber and Lumber
Timber can be divided into two categories - hardwoods and softwoods. There are many types of timber under these two categories. They are-
- Bamboo
- Birch
- Cedar
- Cherry
- Cross-laminated
- Glulam
- Green timber
- Lime
- Mahogany
- Oak
- Pine
- Plywood
- Sapele wood
- Tulipwood
- Walnut
- Wood ash
- Spruce
- Fir
Abrasive, sharp, hard material used to wear away the surface of softer, less resistant materials. Included within the term are both natural and synthetic substances, ranging from the relatively soft particles used in household cleansers and jeweller’s polish to the hardest known material, the diamond. Abrasives are indispensable to the manufacture of nearly every product made today.
Abrasives are used in the form of grinding wheels, sandpapers, honing stones, polishes, cut-off wheels, tumbling and vibratory mass-finishing media, sandblasting, pulp stones, ball mills, and still other tools and products. Only through the use of abrasives is industry able to produce the highly precise components and ultra-smooth surfaces required in the manufacture of automobiles, airplanes and space vehicles, mechanical and electrical appliances, and machine tools.
This article surveys the principal materials used in abrasives, the properties of those materials, and their processing into industrial products. Most abrasive products are made of ceramics, which include some of the hardest materials known. The origins of hardness (and other properties) in ceramic materials are described in the article ceramic composition and properties.
A particle or fibrous which are used in terms of making ceramic products. Ceramics have regular atomic structure and crystal structure. Ceramics are mainly oxides, nitrides and carbides. They are non-conducting materials, due to its insulating property they are used as insulators. They are very hard and brittle in nature.
E.g.: alumina, silica, silicon carbide, diamond, bricks, etc.
Applications:
- Due to the compressive strength bricks are used in construction
- Because of their good thermal insulation ceramic tiles are used in ovens.
- Some ceramics are transparent to radar and other electromagnetic waves are used in radomes and transmitters.
- Glass ceramics have high temperature capabilities so they are used in optical equipment and fibre insulation.
- Alumina, silica, silicon carbide are used in making tools.
- Diamond is used in ornaments and cutting tool applications.
The glass has been used as an engineering material since ancient times. But because of the rapid progress made in the glass industry in recent times, the glass has come out as the most versatile engineering material of the modern times. The first glass objects made by man were of natural glass such as obsidian and rock crystal.
The manufactured glass dates from per-historic times in the Far East, India and Egypt. But its exact place and date of origin are unknown. It is however believed that the ancient Hindus knew the method of glass making long before the Christian era.
With the help of techniques developed in the glass industry, the glass of any type and quality can be produced to suit the requirements of different industries.
Just to stress the importance of glass in the engineering field of today, few of the recent developments that have taken place in the glass industry are mentioned below:
(i) A modern Boeing 707 jet plane contains more than 5000 components of glass.
(ii) The fibre glass reinforced with plastics can be used in the construction of furniture, lampshades, bathroom fittings, navy boats, aeroplanes, cars, trucks, etc.
(iii) The glass is the only material strong enough to go up to the bottom of ocean and to maintain its buoyancy. It is therefore used in the construction of noses of deep-diving vehicles.
(iv)The glass linings are applied on equipment likely to be affected by the chemical corrosion such as valves, pumps, pipes, etc.
(v) In the construction of modern homes, the walls and ceilings of hollow glass blocks can be made. Such construction cuts off the glare. But it admits sunlight and controls sound and heat in a better way.
(vi) In the field of fire-arms, the glass is used to forma rifle barrel which is lighter and stronger than the conventional type.
(vii) It will be interesting to note that now-a-days, it is possible to prepare the colour-changing glass. A window with such glass will be transparent during daytime and it will be a source of light at night.
(viii) The body of a guided missile contains thousands of glass items.
(ix) The development and advancement of sciences of astronomy and bacteriology are mainly due to the use of optical glass.
(x) The mechanical strength of ordinary glass varies from 35 to 70 N/mm2. Due to research in glass industry, it has become possible to produce glass having mechanical strength of about 420 N/mm2.
Classification of Glass:
The glass is a mixture of a number of metallic silicates, one of which is usually that, of an alkali metal. It is amorphous, transparent or translucent. It may also be considered as a solidified super-cooled solution of various metallic silicates having infinite viscosity.
For the purpose of classification, the glass may be grouped into the following five main categories:
(1) Soda-lime glass or commercial glass
(2) Potash-lime glass
(3) Potash-lead glass
(4) Common glass
(5) Borosilicate glass.
Composition of Glass:
The glass is not a single compound. It is therefore very difficult to give any particular chemical formula for it. But with reasonable accuracy, it may generally be expressed as follows –
AX2O, bYO, 6SiO2
Where, a and b are numbers of molecules,
X = an atom of an alkali metal such as Na, K, etc.
Y = an atom of a bivalent metal such as Ca, Pb, etc.
With this expression, the chemical formulas for three groups of glass, as classified above, are as follows:
Properties of Glass:
The properties of glass are mainly governed by factors such as composition of the constituents, state of surface, thermal treatment conditions, dimensions of specimen, etc.
Following are the properties of glass which have made the glass popular and useful:
(i) It absorbs, refracts or transmits light.
(ii) It can take up a high polish and may be used as substitute for very costly gems.
(iii) It has no definite crystalline structure.
(iv) It has no sharp melting point.
(v) It is affected by alkalis.
(vi) It is an excellent electrical insulator at elevated temperatures due to the fact that glass can be considered as an ionic liquid. The ions are not easily moved at room temperature because of the high viscosity. But when the temperature rises, the ions are permitted to flow and thus they will sustain an electric current.
(vii) It is available in beautiful colours.
(viii) It behaves more as a solid than most solids in the sense that, it is elastic. But when the elastic limit is exceeded, it fractures instead of deforming.
(ix) It is capable of being worked in many ways. It can be blown, drawn or pressed. But it is strange to note that it is difficult to cast in large pieces.
(x) It is extremely brittle.
(xi) It is not usually affected by air or water.
(xii) It is not easily attacked by ordinary chemical reagents.
(xiii) It is possible to intentionally alter some of its properties such as fusibility, hardness, refractive power, etc. to suit different purposes.
(xiv) It is possible to obtain glasses with diversified properties. The glasses may be clear, colourless, diffused and stained.
(xv) It is possible to weld pieces of glass by fusion.
(xvi) It is transparent and translucent. The transparency is the most used characteristic of glass and it is due to the absence of free electrons. For the same reason, it also works as a good insulator.
(xvii) When it is heated, it becomes soft and soft with the rise in temperature. It is ultimately transformed into a mobile liquid. This liquid, when allowed to cool, passes to all the degrees of viscosity.
This property of glass has made its manufacturing process easy. It can also be formed into articles of desired shape. Thus, the amorphousness of glass permits it to be blown, drawn from furnaces and continuously worked.
(xviii) Due to advancement made in the science of glass production, it is possible to make glass lighter than cork or softer than cotton or stronger than steel. The strength of glass however is considerably affected by foreign inclusions, internal defects and cords or chemically heterogeneous areas.
(xix) The glass panes can be cleaned easily by any one of the following methods:
(a) Applying methylated spirit;
(b) Painting the glass panes with lime-wash and leaving it to dry and then washing with clean water;
(c) Rubbing damp salt for cleaning paint spots; and
(d) Rubbing finely powdered chalk.
It can thus be easily appreciated that glass, though used for thousands of years, is just beginning to be understood and it is still possible to get a variety of glasses with certain chemical additives. Further investigations are yet in the process for preparing glass with extraordinary unusual characteristics and thus to increase the utility of this unique and complex material.
As a matter of fact, the glass industry has made enormous progress all over the world and the glass has become very cheap and useful to the poor as well as to the rich.
Types of Glass:
The properties and uses of the following types of glass will now be discussed:
(1) Soda-lime glass or commercial glass
(2) Potash-lime glass
(3) Potash-lead glass
(4) Common glass
(5) Borosilicate glass.
(1) Soda-Lime Glass or Commercial Glass:
This is also known as the soda-glass or soft-glass. It is mainly a mixture of sodium silicate and calcium silicate.
Properties:
Following are the properties of soda-lime glass:
(i) It is available in clean and clear state.
(ii) It is cheap.
(iii) It is easily fusible at comparatively low temperatures.
(iv) It is possible to blow or to weld articles made from this glass with the help of simple sources of heat.
Uses:
It is used in the manufacture of glass tubes and other laboratory apparatus, plate glass, window glass, etc.
(2) Potash-Lime Glass:
This is also known as the Bohemian-glass or hard-glass. It is mainly a mixture of potassium silicate and calcium silicate.
Properties:
Following are the properties of potash-lime glass:
(i) It fuses at high temperatures.
(ii) It is not easily affected by water and other solvents.
(iii) It does not melt so easily.
Uses:
This glass is used in the manufacture of glass articles which have to withstand high temperatures such as combustion tubes, etc.
(3) Potash-Lead Glass:
This is also known as the flint glass. It is mainly a mixture of potassium silicate and lead silicate.
Properties:
Following are the properties of potash-lead glass:
(i) It fuses very easily.
(ii) It is easily attacked by aqueous solutions.
(iii) It possesses bright lustre and great refractive power.
(iv) Its specific gravity is about 3 to 3.30.
(v) It turns black and opaque, if it comes into contact with reducing gases of the furnace during heating.
Uses:
It is used in the manufacture of artificial gems, electric bulbs, lenses, prisms, etc.
(4) Common Glass:
This is also known as the bottle glass. It is prepared from cheap raw materials. It is mainly a mixture of sodium silicate, calcium silicate and iron silicate.
Properties:
Following are the properties of common glass:
(i) It fuses with difficulty.
(ii) It is brown, green or yellow in colour.
(iii) It is easily attacked by acids.
Uses:
It is mainly used in the manufacture of medicine bottles.
(5) Borosilicate Glass:
Most of us are more familiar with this type of glass in the form of ovenware and other heat-resisting ware, better known under the trade name Pyrax. Borosilicate glass is made mainly of 70% to 80% silica and 7% to 13% boric oxide with smaller amounts of the alkalis (sodium and potassium oxides) and aluminium oxide.
Properties:
Following are the properties of borosilicate glass:
(i) It has a relatively low alkali content and consequently has good chemical durability and thermal shock resistance.
(ii) It has high softening point.
(iii) It does not break when temperature changes quickly.
Uses:
This glass is widely used in the chemical industry, for laboratory apparatus, for ampoules and other pharmaceutical containers, for various high intensity lighting applications and as glass fibres used in the reinforced plastics to make protective helmets, boats, piping, car chassis, ropes, car exhausts and many other items and also in textile industry.
Treatment of Glass:
The glass may be given any of the following treatment:
(1) Bending
(2) Cutting
(3) Opaque making
(4) Silvering.
(1) Bending:
The glass may be bent into desired shape by placing it in ovens in which the temperature can be regulated. The glass in the form of rods, sheets or tubes is placed in such ovens and heated. It is then bent when it is suitably heated.
(2) Cutting:
The glass is cut in required sizes with the help of diamond or rough glasses or small wheels of hardened steel.
(3) Opaque Making:
The glass can also be made opaque or impervious to light. It is done by grinding the glass surface with emery. It can also be achieved chemically by the application of hydrofluoric acid.
(4) Silvering:
This process consists in applying a very thin coat of tin on the surface of glass. The silver is deposited on this layer of tin. A suitable paint is then applied to give protection against the atmospheric effects.
The term graphite is derived from the Greek word “graphein,” which means to write. The material is typically grayish-black in color, opaque, and has a radiant black sheen. Graphite is a distinct material as it displays the properties of both a metal and a non-metal.
Although graphite is flexible, it is not elastic and has high electrical and thermal conductivity. It is also chemically inert and highly refractory. Since graphite displays low adsorption of X-rays and neutrons, it is very valuable in nuclear applications.
This uncommon combination of properties is due to graphite’s crystalline structure. The carbon atoms are set hexagonally in a planar condensed ring system. The layers are stacked parallel to each other. The atoms within the rings are bonded covalently, while the layers are loosely linked together by van der Waals forces. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions.
For example, graphite’s ability to develop a solid film lubricant is the outcome of these two contrasting chemical bonds. As weak Van der Waals forces control the bonding between each layer, they can slide against one another, making graphite an ideal lubricant. In 2000, worldwide graphite production was estimated to be about 602,000 tons, with China as the largest producer followed by India, Mexico, Brazil, and the Czech Republic.
Graphite Classifications
Graphite can be divided into two main types—natural and synthetic.
Natural Graphite
Natural graphite is a mineral composed of graphitic carbon. It varies considerably in crystallinity. Most of the commercial (natural) graphite’s are mined, and typically contain other minerals. After graphite is mined, it usually requires a considerable amount of mineral processing like froth flotation to concentrate the graphite.
Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °C.
There are three types of natural graphite:
- High crystalline
- Amorphous
- Flake
Crystalline Graphite
It is said that crystalline vein graphite came from crude oil deposits that have transformed into graphite through time, temperature, and pressure. Vein graphite fissures typically measure between 1 cm and 1 m in thickness and usually have a purity of more than 90%.
Although this type of graphite can be found globally, only Sri Lanka commercially mines it, using conventional shaft or surface mining techniques.
Amorphous Graphite
Amorphous graphite is the least graphitic among the natural graphite’s. However, the term “amorphous” is incorrect as the material is still crystalline. Amorphous graphite can be found as minute particles in beds of mesomorphic rocks such as coal, slate, or shale deposits. The graphite content varies from 25% to 85% according to the geological environment.
Conventional mining techniques are used to extract amorphous graphite, which occurs mainly in Mexico, North Korea, South Korea, and Austria.
Flake Graphite
Flake graphite can be found in metamorphic rocks evenly spread through the body of the ore or in concentrated lens-shaped pockets. The range of carbon concentrations varies from 5% to 40%. Graphite flake can be found as a lamella or scaly form in specific metamorphic rocks such as limestone, gneisses, and schists.
Froth flotation is used to extract flake graphite. “Floated” graphite has 80%–90% graphite content. Over 98% of flake graphite is made using chemical beneficiation processes. Flake graphite can be found in numerous places worldwide.
Synthetic Graphite
Synthetic graphite can be produced from coke and pitch. Although this graphite is not as crystalline as natural graphite, it is likely to have higher purity. There are basically two types of synthetic graphite. One is electrographite, pure carbon produced from coal tar pitch and calcined petroleum coke in an electric furnace. The second is synthetic graphite, produced by heating calcined petroleum pitch to 2800 °C.
Essentially, synthetic graphite has higher electrical resistance and porosity, and lower density. Its enhanced porosity makes it unsuitable for refractory applications.
Synthetic graphite contains mainly graphitic carbon that has been attained by graphitization, heat treatment of non-graphitic carbon, or chemical vapor deposition from hydrocarbons at temperatures over 2100 K.
Diamond is a form of carbon that is crystallized in a cubic structure with each carbon atom linked by a strong, rigid chemical bond to four other atoms.
Until the 1950s, diamond was available in relatively small quantities at fairly high prices, the word creating an image of brilliant gemstones and wealth. The broad range of valuable properties has driven the development of routes to make synthetic diamonds, which has produced new products and applications.
Sources
Diamond can now be found from several sources:
- Naturally occurring diamond; about 20 tonnes of diamond are mined each year. Half are gem quality and half are industrial quality.
- Synthetic single crystal diamond; about 90 tonnes of diamond are made annually by the high-pressure and high temperature (HPHT) method. Most industrial diamond is made from graphite at pressures of 4.5 to 6.0 GPa and temperatures of 1400 to 1600 °C with the aid of a molten transition metal catalyst. These diamonds are considerably cheaper than natural diamond.
- Polycrystalline diamonds are formed usually by either cementing diamond grains together using metal as a bonding agent or sintering using Boron Carbide as a sintering aid.
- Vapour phase deposition. Both chemical vapour deposition (CVD) and physical vapour phase deposition (PVD) produce thin diamond films. About 10 tonnes of vapour phase grown diamond films are produce annually. Their cost is more than four times that of naturally occurring diamond. However, their application can be justified economically as they are used in thin film form and make a significant difference to component properties.
Key Properties
Diamond provides an impressive combination of chemical, physical and mechanical properties:
- The hardest known material
- Low coefficient of friction
- High thermal conductivity
- High electrical resistivity
- Low thermal expansion coefficient
- High strength
- Broad optical transparency from ultra violet to infra-red
- Resistant to chemical corrosion
- Biologically compatible
Typical physical and mechanical properties are listed in table 1.
Diamond has limitations. It is meta stable at room temperature and pressure, forming a black coat when heated to above 600 °C in oxygen and reverting to graphite when heated in nitrogen at around 1500 °C.
Diamond will react with strong carbide forming metals (i.e. tungsten, tantalum and zirconium). It dissolves in iron, cobalt, manganese, nickel, chromium and the platinum-group metals.
Table 1. Typical physical and mechanical properties for diamond.
Property | Value |
Density (g/cm3) | 3.50 |
Young’s Modulus (GPa) | 1050 |
Bend Strength (MPa) | 850 |
Fracture Toughness K1c (MPa.m0.5) | 3.5 |
Hardness (GPa) | 45 |
Thermal Expansion Coefficient (x 10-6/°C) | 1.1 |
Coefficient of Friction | 0.02 |
Electrical Resistivity (ohm.cm) | >1013 |
Thermal Conductivity (W/mK) | 400 |
Decomposition Temperature in nitrogen (°C) | 1500 |
Applications
The use of diamond has grown enormously since World War 2, exploiting the unique combination of properties and the increased availability of the material as synthesis methods developed. The following applications are covered in more detail below:
Cutting Tools and Wear Components
The properties exploited by these applications are hardness, strength, low thermal expansion coefficient, low friction coefficient and chemical resistivity.
Some of the products in this area include oil drilling bits, rock drill cutters, wire drawing dies, extrusion dies, cutting tool inserts, optical grinding tools, coatings for computer hard discs and coatings for ball bearings.
Either polycrystalline diamond or diamond coatings can be used in this area When using coatings, manufacturers must pay attention to coating adhesion and ensure the coating is uniform and follows the component contours for successful use. The coating cannot be applied to ferrous materials as it will react and dissolve.
Polymers have chain molecule structure of carbon as back bone atoms. They are mainly made up of tough organic materials. They are low density materials and also flexible. In some cases, polymers are not flexible.
Polymers are not only used as structural materials, they can be used as fiber and resins in the matrix of composite materials.
e.g.: polyester as fibers, phenolic and epoxides as resins.
Elastomers are also polymers but they are considered separately due to their specific design for certain purposes like shock and vibration absorption.
Natural polymers:
E.g. : wool, silk, DNA, cellulose, proteins, etc.
Synthetic polymers:
- Thermo plastics
- Thermosetting plastics
E.g.: nylon, polyethylene, polyester, Teflon, epoxy, Bakelite, etc.
Applications:
- Polyethylene is used for making carry bags.
- Polypropylene is used for making high temperature resistance products like feeding bottle.
- Polyether ether ketone and polyethylene ketone are used in mineral water bottle concept.
- Poly carbonate is used to make high performance polymers like transparent polymers
- Polyaniline is a conducting polymer.
- Bakelite used for making insulating materials.