UNIT 3
Electrochemistry and corrosion
Electrochemical cell is a device that generate electrical energy from chemical reactions. The electrochemical cells which generate an electric current are called voltaic cells and those which generate chemical reactions via electrolysis are called electrolytic cells. A diagram detailing the different parts of an electrochemical cell is provided below:
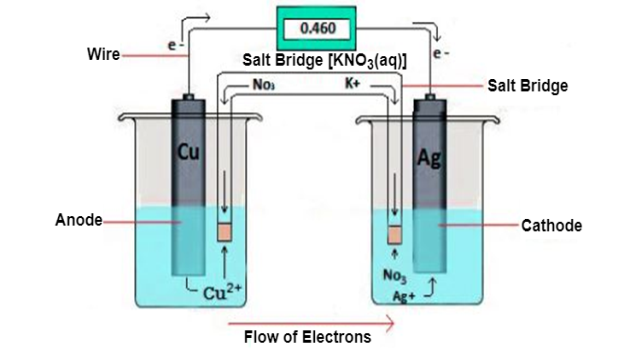
Oxidation half-cell-
M (s)= Mn+ + ne-
Reduction half-cell-
Mn+ + ne_ = M(s)
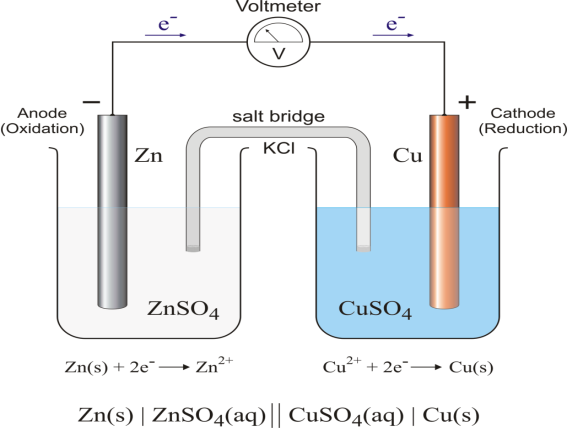
Galvanic cell usually consists two electrolyte solution into which two electrolytes of different metals are dipped.
Principle
The galvanic cell burns with redox reaction, in this cell oxidation and reduction occurs in separate container called half-cell the electron moves from of citation half-cell to reduction half-cell and current flow from reduction half-cell to oxides and half-cell.
The cell is usually representing as
Zn(s)|Zn2+(aq) ||Cu2-|Cu(s)
The two-half reaction are
At anode- (oxidation take place)
Zn Zn2+ + 2 e-
At cathode- (reduction take place)
Cu2+ + 2 e- Cu
Net reaction is- Zn +Cu2- Zn2+ +Cu
Construction –
Electrode Potential:
The electrode potential is defined as the Potential difference developed between the metal ions from metal to the solution or from solution to the metal. At equilibrium the potential difference remains constant. The electrode potential of a metal is defined as the direct measure of its tendency to get reduced is called reduction potential, its value is +x volts. Similarly, the tendency of an electrode to lose electrons is a measure of its tendency to get oxidized is called oxidation potential, its value is –x volts.
Expression of electrode potential:
Consider the following redox reaction
Mn+ + ne- ↔ M
For such a redox reversible reaction, the free energy change (∆ G) and its equilibrium constant (K) are related as;
∆ G = -RT ln k + RT ln [product]/[Reactant]
∆ G0 + RT ln [product]/[Reactant]………………. (i)
Where
∆ G 0 = standard free energy change.
The above equation is known as Van’t Hoff Isotherm.
The decrease in free energy in the reversible reaction will produce electrical energy i.e.
-∆ G = nEF and ∆ G 0 = -nE0F…………………………(ii)
Where
E = Electrode potential
E0 = Standard electrode potential
F = Faraday (96,500 coulombs)
Comparing equation 1 & 2
-nEF = -nE0F + RT ln [M]/[Mn+]
= -nE0F + Rt ln 1/ [Mn+]
Where, concentration of the metal is unity or
-nEF = -nE0F - RT ln [Mn+]
Dividing the equation by –nF
E= E0 + RT ln [Mn+]/nF
E= E0 + 2.303RT log [Mn+]/nF
E= E0 + 0.0591 log [Mn+]/n ……………(iii)
This equation-3 is known as “Nernst Equation” for electrode potential
Calomel Electrode:
Calomel electrode is particularly very simple to construct, free from surface sensitivity and accurate to use even in a very normal laboratory.
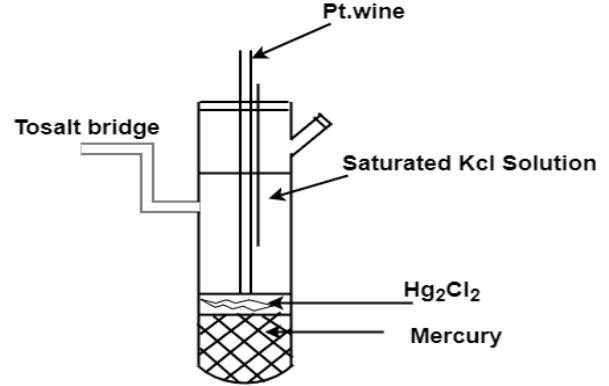
The calomel electrode consists of an inner glass tube and an outer jacket. In the inner glass tube, a platinum wire is dipped into mercury which rests on a paste of mercurous chloride, Hg2Cl2 (commercially known as calomel) and mercury. This paste is in contact with KCl present in the outer jacket, through the glass frit plug fixed at the bottom of inner glass tube. The calomel electrode comes in contact with the experimental solution through a frit arranged to the outer jacket. The potential of this electrode depends on the concentration of KCl taken in the outer jacket.
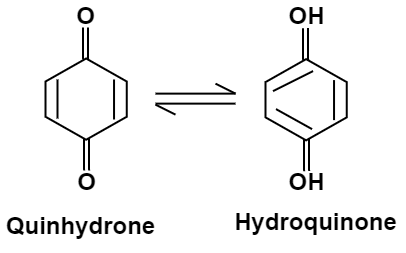
Quinhydrone Electrode:
This is a redox electrode reversible to protons and often replaces the hydrogen electrode. Quinhydrone is a 1:1 molar mixture of quinone and hydroquinone. The electrode consists of a shiny platinum electrode dipped in an acid / base test solution, which is saturated with quinhydrone.
Advantages:
- The quinhydrone electrode is simple to set up and needs no removal of air.
- The reversibility equilibrium is achieved faster than hydrogen gas electrode thereby allowing a quicker measurement.
- PH values of solutions containing reducible substances like Cu2+, Cd2+, unsaturated acids, NO3 -, etc., and catalytic poisons can be measured using quin-hydrone electrode.
Limitations:
- The electrode cannot be used at pH values greater than 8.
- This electrode also fails in the presence of strong oxidizing and reducing agents.
Glass electrode:
Most often used pH electrodes are called glass electrodes and belong to the family of ISE. They are sensitive only to H+ ions. Typical glass electrode is made of glass tube engaged with small glass bubble sensitive to protons. Inside of the electrode is usually filled with buffered solution of chlorides in which silver wire covered with silver chloride is immersed.
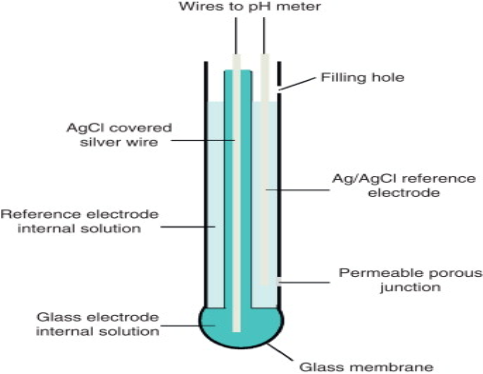
PH of internal solution varies- E.g.; it can be 1.0(0.1M HCl) or 7.0 Active part of the electrode is the glass bubble. While tube has strong and thick walls, bubble is made to be as thin as possible. Surface of the glass is protonated by both internal and external solution till equilibrium is achieved. Both sides of the glass are charged by the adsorbed protons, this charge is responsible for potential difference. This potential in turn is described by the Nernst equation and is directly proportional to the pH difference between solutions on both sides of the glass. The majority of pH electrodes available now a day are combination electrodes that have both glass H+ ion sensitive electrode and reference electrode compartments, conveniently placed in one housing.
This equation is named after the name of scientist who discovered is Walther Nernst. Nernst equation plays a major role in relating the Reduction Potential with the electrode potential, temperature of the chemicals which are undergoing the oxidation or reduction. (Reduction Potential is used to measure the tendency of the chemical species to acquire or loose electron to an electrode.)
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transforms into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation ………. (i)
 G=-nFE ………. (ii)
G=-nFE ………. (ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE* …………. (iii)
G*=-nFE* …………. (iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ …………. (iv)
G= G*+RT lnQ …………. (iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ ……………. (v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ (6) ………. (vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q ……. (vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q ……. (viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the …. (potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the …. (potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting these values to Nernst Equation we get,
0=E*-RT/nF In K ……. (ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Electrochemical:
The deterioration of materials by chemical process is called as corrosion. In electrochemical corrosion M→M+ + e- is facilitated by the presence of suitable electron acceptor and some time it is also called as depolarizer. Corrosion can also be viewed as the spontaneous return of metals to their ores, the abandoned amount of energy used that were consumed in the mining, refining into useful objects is dissipated by variety of different routes.
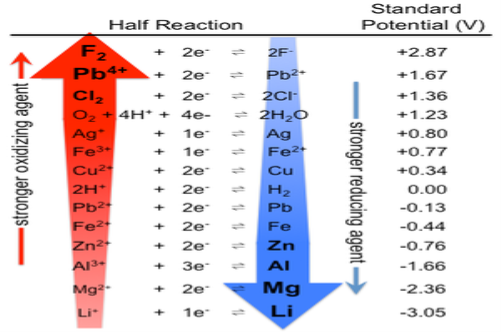
Corrosion Cells and reactions:
The occurrence of oxidation and reduction steps in corrosion results in separation of metal’s location. This can be possible due to the conductive property of metal, so that electrons can flow through metal form anodic to cathodic regions. The water presence plays a major role on transportation.
Fe → Fe2+ + 2e-
The metal is under pressure or is isolated from the air. The metal ions dissolve in the moisture film and e- migrates to another location.
The anodic process is
Fe(s) → Fe2+ (aq) + 2e-
While cathodic steps involve the reduction of oxygen gas
O2 + 2H2O (aq) + 4e- → 4OH-
Or the proton reduction
H+ + e- → ½ H2(g)
Or the reduction of metal ion
M2+ + 2e- → M(s)
Since both the cathodic and anodic steps must take place for corrosion to occur, prevention of either one will stop corrosion. The most obvious strategy is to stop both processes by coating the object with a paint or other protective coating. Even if this is done, there are likely to be places where the coating is broken or does not penetrate, particularly if there are holes or screw threads. A more sophisticated approach is to apply a slight negative charge to the metal, thus making it more difficult for the reaction to take place:
M⟶M2++2e−.
Applications:
- The thermal stability of the metal oxide depends on its electropositive nature. As the electro positivity decreases from top to bottom, the thermal stability of the oxide also decreases from top to bottom.
- A more electropositive metal can displace a less electropositive metal from its salt's solution.
- The metal which can provide electrons to ions present in dilute acids for reduction, evolve hydrogen from dilute acids.
The measurement of suitable indicator electrode with respect to the reference electrode as a function of titrant volume is called as the potentiometric titrations. It provides more accurate data with respect to the data of chemical indicators. These are useful for the detection of the presence of unsuspected species.
Batteries:
The electrical interconnection of two or more electrochemical cells, each of which contain two electrodes and an electrolyte is called a Battery. The condition at which battery is properly working is supply of electric power, the positive terminal is cathode while the negative terminal is anode.
Primary (Lithium cell)
It consists of lithium anode with solid electrolyte or liquid electrolyte and solid or liquid cathode. A thin protective insulating film is formed on lithium anode protecting the anode against corrosion as it is conductive to lithium ions but not electrons while water and alcohol never form such film.
Lithium iodide solid cathode cell consists of iodine PVP cathode with 3V voltage. It is highly stable and dependable and hence used in medical source for electronic flash guns of cameras.
Lithium Ion Cells
Anode: Graphite, Carbon compound.
Cathode: Oxide of Lithium
Uses:
Used in Laptops, cellular phones, electronic vehicles.
Secondary Batteries:
Lead-Acid Storage Battery & Lithium Ion Battery:
Lead storage battery is the most common device used to store energy in the portable form. This is also called as lead acid battery. Although the batteries are reliable, which contain acidic material inside that required a proper disposal method after its complete use. These batteries have moderate power density and good time. The battery consists of lead grids on its electrodes. The anodic grid opening is filled with spongy lead while the cathodic grid consists of lead oxide (PbO2).
Charge Chemistry of the battery:
Charge batteries are those batteries which can be recharged after single use. In this type of battery each plate contains negative as well as the positive end. The negative plate is of lead while the positive plate is made up of lead oxide in an electrolyte of approx. 4.0M sulphuric acid.
Negative plate reaction:
PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO4–(aq)
Positive plate reaction:
PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e–
Combining these two reactions, the overall reaction is the reverse of the discharge reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq)
Discharge Chemistry of the Battery:
The positive and negative plate of the batteries becomes lead sulphate. Due to the loss of sulfuric acid from electrolytes it becomes the water.
Negative plate reaction:
Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2e–
Positive plate reaction:
PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Combining these two reactions, one can determine the overall reaction:
Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq) → 2PbSO4(s) + 2H2O(l)
Causes of Corrosion:
Corrosion is the electrochemical process that occurs in various forms such as chemical forms and atmospheric forms. On the contact of acid substance with iron it pretends to form rust. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture. When steel is exposed to water, the iron particles are lost to the water’s acidic electrolytes. The iron particles then become oxidized, which results in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and flow through the steel to another area of the steel known as the cathodic area.
Oxygen causes these electrons to rise up and form hydroxyl ions (OH). The hydroxyl ions react with the Fe2+ to form hydrous iron oxide, better known as rust. Where the affected iron particles were, has now become a corrosion pit, and where they are now, is called the corrosion product (rust).
Consequences of corrosion:
Corrosion has many consequences on economic, health, safety, to our society.
Economic Effects: To determine the economic cost on the country’s economy there are several studies conducted by different country. Among all the most extensive study was carried out by United State in 1975 and gathered that it cost about $70 billion.
Health Effects: Recent years have seen an increasing use of metal prosthetic devices in the body, such as pins, plates, hip joints, pacemakers, and other implants. New alloys and better techniques of implantation have been developed, but corrosion continues to create problems. E.g.- inflammation caused by corrosion products in the tissue around implants, fracture of weight-bearing prosthetic devices.
Technological effects: Technology sector is also very badly affected by the corrosion. A great deal of the development of new technology is held back by corrosion problems because material is required to withstand in many cases simultaneously higher temperature, higher pressure. Corrosion problems that are less difficult to solve affect solar energy systems, which require alloys to withstand hot circulating heat transfer fluids for long periods of time, and geothermal systems, which require materials to withstand highly concentrated solutions of corrosive salts at high temperatures and pressures.
Chemical Corrosion: The reaction of metal with water vapour or gas at high temperature causes the metal to corrode chemically. This is the redox process in which the electron of the metal is passed directly to the substance in the environment. The metal corrodes generally in the metal which is in higher contact with water.
 3Fe + 4H2O Fe3O4 + 4H2
3Fe + 4H2O Fe3O4 + 4H2
 3Fe + 2O2 Fe3O4
3Fe + 2O2 Fe3O4
Electrochemical Corrosion: Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
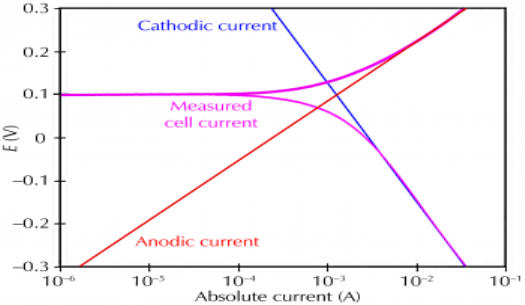
Corrosion process showing the anodic and cathodic component of current
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
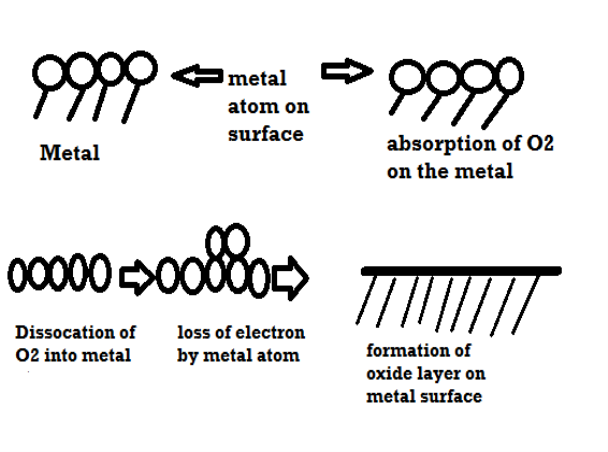
There are several types of corrosion:
(i) Galvanic Corrosion: Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrode.
(ii) Pitting Corrosion: This type of corrosion occurs at certain conditions, there is an accelerated corrosion in some areas rather than the uniform corrosion over the substance. This condition includes low level of concentration of oxygen or high concentration of chlorides.
(iii) Microbial Corrosion: Microbial Corrosion is caused by micro-organisms. They commonly referred to as microbiologically influenced corrosion. It applies to both metallic and non-metallic materials with or without oxygen. In the presence of oxygen there are some bacteria that directly oxidize iron to iron oxides and hydroxides while in the absence of oxygen sulphate reducing bacteria are active and produce hydrogen sulphide causes sulphide stress cracking.
(iv) High Temperature Corrosion: The deterioration caused on the metal due to heating. This can cause when the metal is kept in hot atmosphere that too in the presence of oxygen, sulphur, or with any other compound which is capable of oxidizing the material.
(v) Crevice Corrosion: This occurs in confined spaces where access of fluid from the environment is limited such as gaps and contact areas between parts, under gaskets or seals, inside cracks and seams and spaces filled with deposits.
(i) Nature of metal
Position of metal in galvanic series.
If position is higher in galvanic series then it corrodes faster
While for 2 metal the difference between them shows the corrosion ratio.
(ii) Potential Difference
If the difference at the electrode potential between two metal is high then the rate of corrosion would be also high while vice versa for lesser difference.
(iii) Purity of metal
Corrosion never took place in pure metals. While if metal itself has an impurity then galvanic cell set up easily which intend increases the rate of corrosion.
(iv) Relative areas of cathode and anode parts
Rate of corrosion is directly depending on the area of cathode and inversely depends on the area of anode. If the area of cathode is larger then there is more demand of electrons while in the smaller anode area the corrosion took place very fast.
(v) Nature of corrosion Product
Metal oxide film is formed on the surface of metal by corrosion due to oxygen. The formed film would be stable, unstable, and volatile.
(vi) Temperature
At high temperature the rate of corrosion increases as because there is a consistent increase in the ionization and mobility difference rate while in some cases rate of corrosion decreases at high temperature as the solubility of O2 gas increases.
(vii) Presence of moisture
The rate of corrosion decreases in dry while increases in presence of moisture. Moisture act as the solvent for setting up of electrochemical corrosion.
(viii) Effect of pH
Rate of corrosion is high at acidic pH due to the evolution of H2 gas at cathode.
(ix) Concentration of electrolytes
This is also called as the Oxygen concentration cell. The rate of corrosion would be directly depending on the supply of oxygen on air.
(x) Over Voltage
The difference between the actual value and theoretical value of decomposition potential of electrode.
Cathodic Protection:
This is the technique used to control the corrosion on the surface of metal by formation of cathode layer on an electrochemical cell. There are 2 types of cathodic protections:
(i) Sacrificial Anodic Protection
(ii) Impressed Current Cathodic Protection
Sacrificial Anodic Protection:
The metal surface can be protected from the corrosion by connecting it wire to a more anodic metal. The sacrifice of this more anodic metal to save the metal form corrosion is called as the Sacrificial Anode. The most common metal used for this purpose are Mg, Zn, Al etc.
Applications:
(i) The underground cable and pipeline protection from soil erosion.
(ii) Ships and boat protection from marine corrosion.
(iii) Prevention of rusty water by inserting Mg sheets or rods into domestic water boiler or tanks.
Impressed Current Cathodic Protection:
This is the type of corrosion protection which consist of sacrificial anodes that is connected to an external power source. The external power source is DC power supply that provides the sufficient current to drive electrochemical reaction required for the cathodic protection to occur.
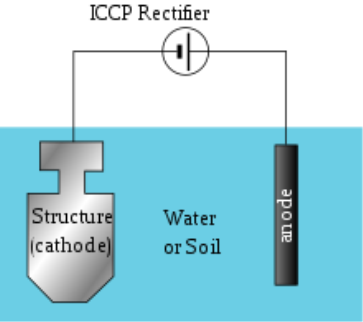
Surface coatings:
This type of coating can mitigate the adverse effects of nanoparticles, can avoid agglomerations, and can prevent the dissolutions and release of toxic ions. The coating up of the surface in order to retain it from the corrosions.
Metallic coatings:
This is used to separate the metal surface from the corrosive environment. This metal coating can be classified into two groups i.e.; more active than the base metal and those that are more noble. To protect the steel surface the first group includes coatings of zinc, aluminum and somewhat of cadmium. These coatings act as sacrificial node at the discontinuity sites and thus afford cathodic protection to the metal. The lifetime of coated structures is often related to the thickness of coating.
Applications:
(i) The coating up of metals is protecting the substrates from being corrode.
(ii) Waterproofing up of woods.
(iii) Sealing the concrete surfaces.
(iv) Waterproofing and damp proofing of the concrete walls.
(v) Coating up of the roof.
(vi) Sealing and waterproofing of masonry.
Electroless plating of Nickel:
The process by which formation of Nickel layer took place on the base metal or the polymer. The strong reducing agent plays a major role in the formation of Nickel layer. The base layer must be treated with the organic solvent followed by the acid. The considered substrate is non-conductor, polymer etc.
Used Metal Ion Solution: Nickel Chloride (NiCl2)
Reducing Agent: NaPO2H2
Buffer solution: CH3COONa (used to maintain the pH)
Complexing Agent: C4H4Na2O4
PH: 4.5
Temperature: 93oC
 At Cathode Ni2+ + 2e- Ni
At Cathode Ni2+ + 2e- Ni
 At Anode H2PO2- + H2O H2PO3- + 2H+ + 2 e-
At Anode H2PO2- + H2O H2PO3- + 2H+ + 2 e-
 Ni2+ + H2PO2- + H2O Ni + H2PO3- + 2H+
Ni2+ + H2PO2- + H2O Ni + H2PO3- + 2H+
Applications:
(i) Automotive industry in bumpers, rims, exhaust pipes
(ii) Bright work on bicycles.
(iii) Used as connectors for electronic devices - mobile phones