Unit - 2
Pressures and Head
A normal force exerted by a fluid per unit area is defined as pressure. Only when dealing with a gas or a liquid do we use the term "pressure." Normal stress is the solids' analogue to pressure. Because pressure is defined as force per unit area, it is measured in newtons per square metre (N/m2), also known as a pascal (Pa).
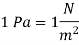
The pressure unit pascal is too small for pressures encountered in practice. Therefore, its multiples kilopascal and megapascal are commonly used.
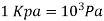
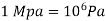
Bar, standard atmosphere, and kilogram-force per square centimetre are three more pressure units often used in practise, particularly in Europe:
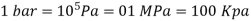
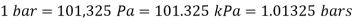
For solids, pressure is used interchangeably with normal stress, which is force acting perpendicular to the surface per unit area.
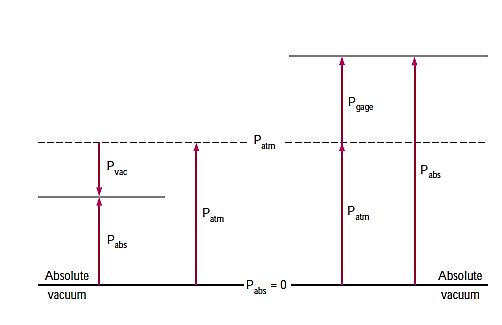
The absolute pressure is the actual pressure at a specific location, and it is measured in comparison to absolute vacuum (i.e., absolute zero pressure).
Most pressure-measuring instruments, on the other hand, are calibrated to read zero in the atmosphere and so show the difference between absolute and local atmospheric pressure. The gauge pressure is the name given to this disparity.
Vacuum pressures are pressures below atmospheric pressure that are measured using vacuum gauges that show the difference between atmospheric and absolute pressure. Absolute, gauge, and vacuum pressures are all positive values that are proportional to one another.
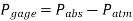
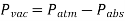
Key Takeaway:
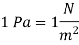
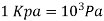
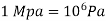
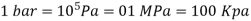
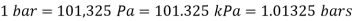
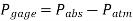
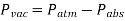
The compressive force per unit area is known as pressure, and it appears to be a vector. Pressure in a fluid, on the other hand, is constant in all directions. It is a scalar quantity since it contains magnitude but not a definite direction. Consider a small wedge-shaped fluid element of unit length (into the page) in equilibrium, as illustrated in the diagram.
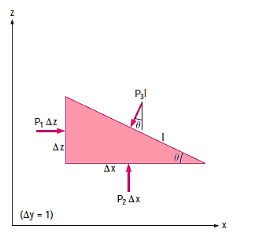
The mean pressures at the three surfaces are
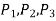
The force acting on a surface is the product of mean pressure and the surface area.
From Newtons 2nd Law a force balance,
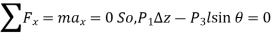
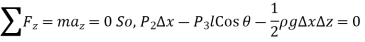
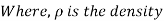
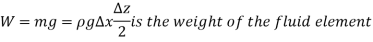
The wedge is a right triangle,
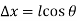
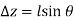
Substituting these geometric relations and dividing by 
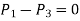
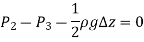
As, 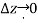 and the fluid element decreases to a point when the wedge gets minuscule. The findings of these two relations are then combined to yield
and the fluid element decreases to a point when the wedge gets minuscule. The findings of these two relations are then combined to yield
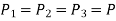
Regardless of the angle 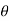
A similar conclusion can be obtained by repeating the analysis for an element in the xz-plane. As a result, we can deduce that the pressure at a given place in a fluid is the same in all directions. It can be demonstrated that this result holds true for fluids in motion as well as fluids at rest in the absence of shear forces.
Key Takeaway:
Pressure in a fluid at rest does not change in the horizontal direction, which should come as no surprise. Consider a thin horizontal layer of fluid and do a force balance in any horizontal direction to demonstrate this. In a gravity field, however, this is not the case in the vertical direction.
Because more fluid rests on deeper layers, pressure in a fluid rises with depth, and the effect of this "additional weight" on a deeper layer is countered by an increase in pressure.
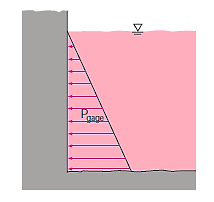
Consider a rectangular fluid element to obtain a relationship for the fluctuation of pressure with depth.
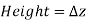
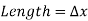
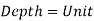
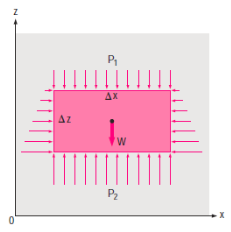
Assuming the density to be constant,
A force balance gives,
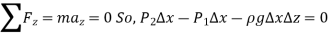
Were,
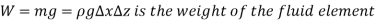
Dividing by  and rearranging gives,
and rearranging gives,
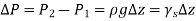
Were,
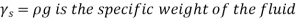
As a result, we may deduce that the pressure differential between two places in a constant density fluid is proportional to the vertical distance 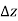 between them and the fluid's density
between them and the fluid's density 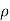 .
.
In other words, pressure in a fluid rises in direct proportion to depth. This is what a diver experiences when diving deeper in a lake. For a given fluid, the vertical distance 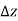 is sometimes used as a measure of pressure, and it is called the pressure head.
is sometimes used as a measure of pressure, and it is called the pressure head.
Key Takeaway:

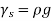
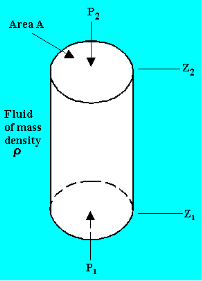
Consider the case of a hypothetical differential cylindrical fluid element.
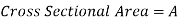
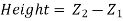
Upward Force due to pressure 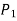 on the element
on the element 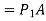
Downward Force due to pressure 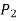 on the element
on the element 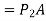
Force due to weight of the element 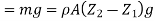
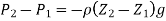
As a result, pressure falls in any fluid subjected to gravitational acceleration as height z increases in the upward direction.
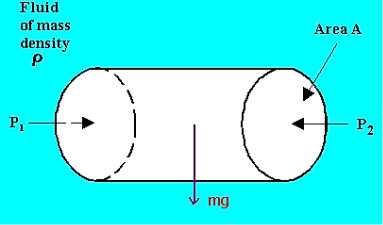
Equating the horizontal forces,
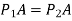
(i.e., some of the horizontal forces must be zero)
Key Takeaway:
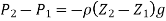
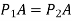
We also find that, due to their low density, the variation of pressure with height for gases is insignificant across short to moderate distances. Because the weight of the gas is too little to make a substantial impact, the pressure in a tank containing a gas, for example, can be called uniform. Furthermore, the pressure in an air-filled space can be assumed to be constant.
If we consider point 1 to be the free surface of a liquid open to the environment, with atmospheric pressure 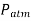 , the pressure at a depth h from the free surface becomes
, the pressure at a depth h from the free surface becomes
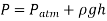
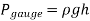
Because liquids are virtually incompressible, the variation un density with depth is minimal. When the height shift isn't too great, this is also true for gases. The difference in density of liquids or gases with temperature, on the other hand, can be large and should be considered when high accuracy is required. Also, because of the large quantity of liquid weight above a liquid at great depths, such as those found in oceans, the change in density of a liquid can be significant.
A relationship for the variation of pressure with height may be established for fluids whose density changes significantly with elevation by dividing the force balance equation by  and taking the limit
and taking the limit 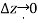
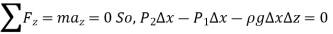
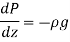
Because pressure decreases in an upward direction, we take the positive z direction to be upward, causing 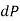 to be negative when
to be negative when 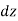 is positive.
is positive.
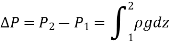
For constant density and constant gravitational acceleration, this relation reduces to
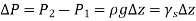
The form or cross section of the container has no effect on the pressure in a fluid at rest. It varies with vertical distance but is constant in all other directions. As a result, the pressure in a given fluid is the same at all points on a horizontal plane.
Key Takeaway:
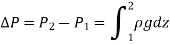
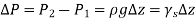
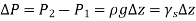
Were,
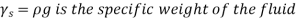
As a result, we may deduce that the pressure differential between two places in a constant density fluid is proportional to the vertical distance 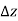 between them and the fluid's density
between them and the fluid's density 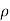 .
.
In other words, pressure in a fluid rises in direct proportion to depth. This is what a diver experiences when diving deeper in a lake. For a given fluid, the vertical distance 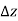 is sometimes used as a measure of pressure, and it is called the pressure head.
is sometimes used as a measure of pressure, and it is called the pressure head.
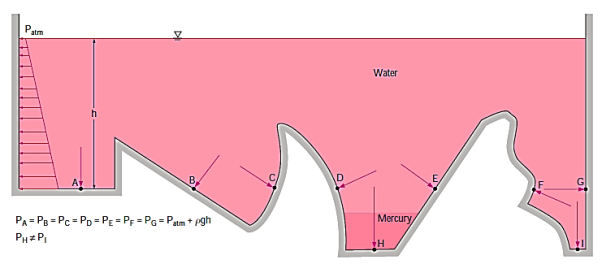
Because they are at the same depth and are connected by the same static fluid, the pressures at points A, B, C, D, E, F, and G are the same.
However, although being at the same depth, the pressures at locations H and I are not the same since these two points cannot be interconnected by the same fluid (i.e., we cannot construct a curve from point I to point H while always remaining in the same fluid). (Can you figure out where the pressure is higher?) Furthermore, the fluid's pressure force is always normal to the surface at the indicated places.
Because the pressure in a fluid remains constant in the horizontal direction, applying pressure to a confined fluid increases the pressure by the same amount throughout. This is known as Pascal's law. Pascal also understood that a fluid's force is related to its surface area. He understood that by connecting two hydraulic cylinders of various sizes, the larger might be used to exert a proportionally greater force than the smaller. Many technologies that are part of our daily lives, such as hydraulic brakes and lifts, can be traced back to "Pascal's machine."
This is what allows us to simply raise a car with just one arm.
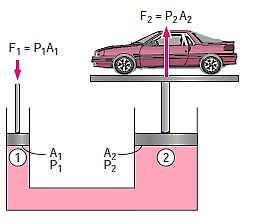
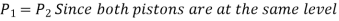
The output force to input force ratio is determined to be (the influence of small height changes is insignificant, especially at high pressures)
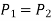
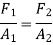
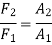
The area ratio A2 /A1 is called the ideal mechanical advantage of the hydraulic lift.
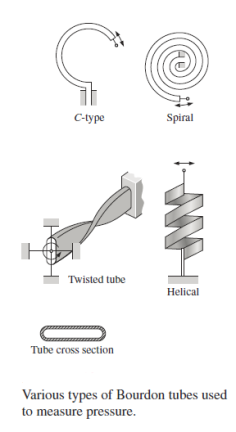
Bourdon Tube:
The Bourdon tube, named after the French engineer and inventor Eugene Bourdon (1808–1884), is a mechanical pressure measurement device that consists of a hollow metal tube bent like a hook whose end is closed and attached to a dial indicator needle. The tube is undeflected when it is open to the atmosphere, and the needle on the display is calibrated to read zero at this point (gage pressure). The tube stretches and moves the needle in accordance with the pressure applied when the fluid inside the tube is pressured.
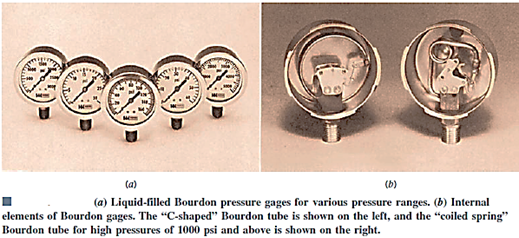
Despite their widespread use, manometers are not well suited to detecting extremely high pressures or pressures that change rapidly over time. They also necessitate the measuring of one or more column heights, which, while not difficult, can be time-consuming. Other types of pressure-measuring tools have been developed to address some of these issues. The majority of these are based on the premise that when a pressure is applied to an elastic structure, the structure will deform, and the size of the deformation may be connected to the pressure. The Bourdon pressure gauge is probably the most well-known example of this type of gadget.
The hollow, elastic curved tube Bourdon tube, which is coupled to the pressure source as indicated, is the most important mechanical element in this gauge. The tube straightens as the pressure inside it rises, and although though the deformation is slight, it may be translated into the motion of a pointer on a dial, as shown. The indicated pressure is gauge pressure because it is the difference in pressure between the outside of the tube atmospheric pressure and the inside of the tube that causes the tube to move.
The Bourdon gauge must be calibrated so that the dial reading accurately indicates the pressure in psi, psf, or pascals. When the gauge reads zero, it means the recorded pressure is the same as the local atmospheric pressure. A negative gauge pressure( vacuum) as well as positive pressures can be measured with this type of gauge.
Another form of mechanical gauge for detecting atmospheric pressure is the aneroid barometer. The traditional Bourdon gauge is not adequate for this measurement because atmospheric pressure is reported as an absolute pressure. The common aneroid barometer is made up of a hollow, closed, elastic element that has been evacuated to near absolute zero pressure. The element deflects as the external atmospheric pressure varies, and this motion can be translated into the movement of an attached dial. The dial, like the Bourdon gauge, can be calibrated to give direct atmospheric pressure readings in millimetres or inches of mercury.
Manometer:
As,
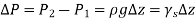
Above equation suggests that a fluid column can be used to measure pressure differences.
A manometer is a gadget that works on this concept and is widely used to monitor minor and moderate pressure variations. A manometer is essentially a glass or plastic U-tube filled with one or more fluids, such as mercury, water, alcohol, or oil. If substantial pressure variations are expected, heavier fluids such as mercury are utilised to keep the size of the manometer to a bearable level.
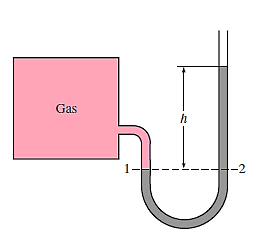
Consider the manometer in the image, which is used to gauge the tank's pressure. The pressure in the tank and at position 1 is the same since the gravitational effects of gases are insignificant. Furthermore, because pressure in a fluid does not vary horizontally, the pressure at point 2 is the same as the pressure at point 1.
The static equilibrium differential fluid column of height h is open to the atmosphere. The pressure at point 2 is then calculated using Equation.
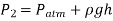
Where 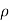 is the fluid density in the tube. It's worth noting that the tube's cross-sectional area has no bearing on the differential height h, and consequently the fluid's pressure.
is the fluid density in the tube. It's worth noting that the tube's cross-sectional area has no bearing on the differential height h, and consequently the fluid's pressure.
However, the tube's diameter should be large enough (at least a few millimetres) to eliminate the surface tension effect and hence the capillary rise.
Multiple immiscible fluids of varying densities piled on top of each other are used in many engineering issues including some manometers. It's easy to study such systems if you recall that
- The pressure change over a fluid column of height h is
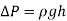
- Pressure increases downward in each fluid and decreases upward
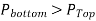
- Two points at the same elevation in a continuous fluid at rest are at the same pressure.
The last concept, which is based on Pascal's law, permits us to "jump" from one fluid column to the next in manometers without worrying about pressure changes as long as we don't jump over another fluid and the fluid remains at rest. The pressure at any place can thus be calculated by starting with a known pressure point and then adding or removing 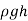 terms as we go closer to the point of interest.
terms as we go closer to the point of interest.
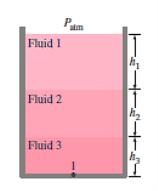
Starting at the free surface, where the pressure is 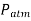 , we can ascertain the pressure at the bottom of the tank by descending downward until we reach point 1 at the bottom and setting the result equal to P1.
, we can ascertain the pressure at the bottom of the tank by descending downward until we reach point 1 at the bottom and setting the result equal to P1.
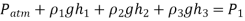
Due to the presence of a device such as a valve or heat exchanger, or any barrier to flow, manometers are particularly well-suited to measuring pressure drops over a horizontal flow section between two specified sites. This is accomplished by connecting the manometer's two legs to these two locations, as indicated.
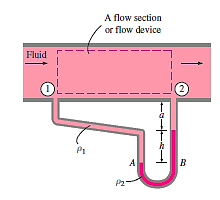
The working fluid can be either a gas or a liquid,
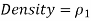
The density of the manometer fluid
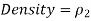
The differential fluid height is h
A relation for the pressure difference 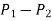 can be obtained by starting at point 1 with
can be obtained by starting at point 1 with 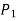 , moving along the tube by adding or subtracting the
, moving along the tube by adding or subtracting the 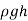 terms until we reach point 2, and setting the result equal to
terms until we reach point 2, and setting the result equal to 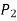 .
.
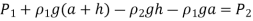
Because the pressure at both sites is the same, we leaped from point A to point B horizontally and ignored the region underneath. Simplifying,
The distance a has no bearing on the outcome, but it must be considered in the analysis. In addition, if the fluid going through the pipe is a gas, then 
The relation simplifies to,
- Case:1:
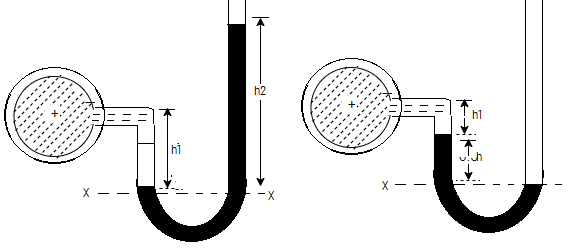
Let A be the point at which pressure is to be measured. X-X is the datum line as shown.
Let, h1 = height of the light liquid in the left limb above the datum line
h2 = height of the heavy liquid in the right limb above the datum line
h = pressure in pipe, expressed in terms of head
S1 = specific gravity of the light liquid, and
S2 = specific gravity of the heavy liquid
The pressure in the left limb and right limb above the datum line X-X are equal.
Pressure head above X-X line in the left limb = h+h1S1
Pressure head above X-X in the right limb = h2S2
Equating these two pressures, we get
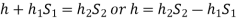
- Case:2:
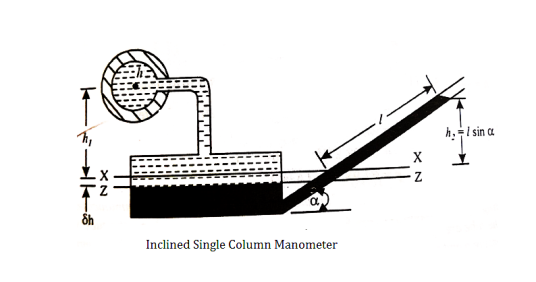
l = length of the heavy liquid moved in right limb,
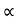 = inclination of right limb horizontal, and
= inclination of right limb horizontal, and
h2 = vertical rise of liquid in right limb from x-x = l sin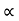
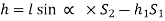
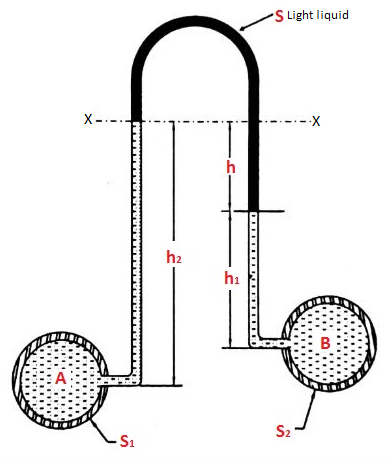
- Case:3:
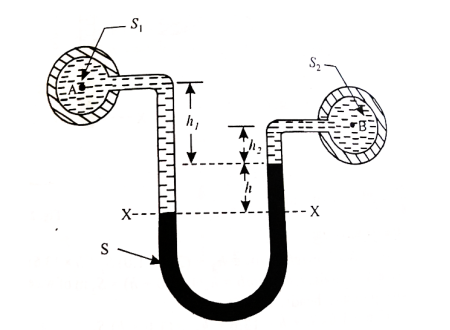
Let, h = difference of mercury level (heavy liquid) in the U-tube,
h1 = distance of the centre of A, from the mercury level in the left limb
h2 = distance of the centre of B, from the mercury level in the right limb,
S1 = specific gravity of liquid in pipe A,
S2 = specific gravity of liquid in pipe B,
S = specific gravity of heavy liquid o mercury
hA = pressure head at A,
hB = pressure head at B
Considering the pressure heads above the datum line x-x, we get
Pressure head in the left limb:
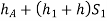
Pressure head in the right limb:
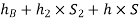
Equating the above pressure heads, we get
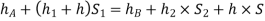
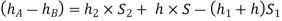
- Case:4:
Let, h = difference of mercury level (light liquid) in the U-tube,
h1 = Height of liquid in the left limb below the datum line X-X
h2 = Height of liquid in the right limb below the datum line X-X
S1 = specific gravity of liquid in pipe A,
S2 = specific gravity of liquid in pipe B,
S = specific gravity of light liquid
hA = pressure head at A,
hB = pressure head at B
Considering the pressure heads below the datum line x-x, we get
Pressure head in the left limb:
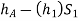
Pressure head in the right limb:
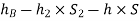
Equating the above pressure heads, we get
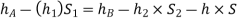
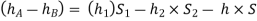
Barometer:
The atmospheric pressure is commonly referred to as the barometric pressure since it is measured by a device called a barometer. Evangelista Torricelli (1608–1647), an Italian, was the first to demonstrate definitively that atmospheric pressure may be measured by inverting a mercury-filled tube into a mercury container open to the atmosphere.
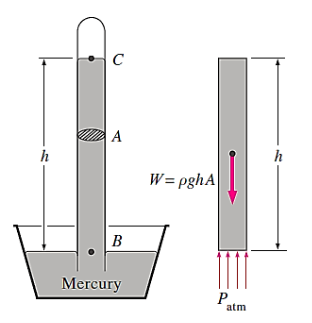
Because there is just mercury vapour above point C and the pressure is relatively low relative to 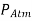 and can be omitted to an excellent approximation, the pressure at C can be taken to be zero. In the vertical direction, write a force balance.
and can be omitted to an excellent approximation, the pressure at C can be taken to be zero. In the vertical direction, write a force balance.
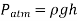
Where 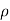 denotes mercury density, g denotes local gravity acceleration, and h denotes the mercury column's height above the free surface. It's worth noting that the tube's length and cross-sectional area have no bearing on the height of a barometer's fluid column.
denotes mercury density, g denotes local gravity acceleration, and h denotes the mercury column's height above the free surface. It's worth noting that the tube's length and cross-sectional area have no bearing on the height of a barometer's fluid column.
The standard atmosphere is a commonly used pressure unit that is defined as the pressure produced by a 760 mm tall column of mercury at 0°C.
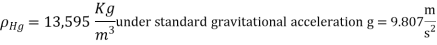
A water column of around 10.3 m would be required to measure the standard atmospheric pressure instead of mercury. Pressure is occasionally described in terms of the height of the mercury column (particularly by weather forecasters).
The standard atmospheric pressure, is
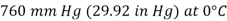
The unit mmHg is also called the torr in honour of Torricelli.
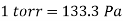
The standard atmospheric pressure 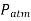 changes from 101.325 kPa at sea level to 89.88, 79.50, 54.05, 26.5, and 5.53 kPa at altitudes of 1000, 2000, 5000, 10,000, and 20,000 meters, respectively.
changes from 101.325 kPa at sea level to 89.88, 79.50, 54.05, 26.5, and 5.53 kPa at altitudes of 1000, 2000, 5000, 10,000, and 20,000 meters, respectively.
Electronics have infiltrated every part of life, including pressure measurement instruments. Pressure transducers, or modern pressure sensors, use a variety of approaches to convert the pressure effect to an electrical effect, such as a change in voltage, resistance, or capacitance. Pressure transducers are smaller and faster than mechanical counterparts, and they can be more sensitive, reliable, and precise. They can measure pressures from less than a millionth of 1 atm to several thousands of atm.
Pressure transducers come in a variety of shapes and sizes to measure gauge, absolute, and differential pressures in a variety of applications. By venting the back side of the pressure-sensing diaphragm to the atmosphere, gauge pressure transducers use atmospheric pressure as a reference, and they deliver a zero-signal output at atmospheric pressure regardless of altitude.
At full vacuum, the absolute pressure transducers are calibrated to produce a zero-signal output. Instead of employing two pressure transducers and subtracting their differences, differential pressure transducers directly measure the pressure difference between two places.
Example:
Bourdon tube is connected to a linear variable differential transformer (LVDT)
The LVDT's core is attached to the free end of the Bourdon tube, so that when pressure is applied, the tube's end pushes the core through the coil, causing an output voltage to form. This voltage is a linear function of pressure, and it can be recorded on an oscillograph or digitised for storage or computer processing.
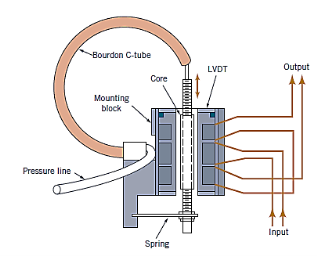
One downside of a pressure transducer that uses a Bourdon tube as the elastic sensing element is that it can only measure static or slowly changing pressures ( quasistatic). The Bourdon tube cannot adapt to quick changes in pressure due to its comparatively large mass.
To circumvent this problem, a different form of transducer is utilised, one with a thin, elastic diaphragm in contact with the fluid as the sensing element. The diaphragm deflects as pressure changes, and this deflection can be measured and translated into an electrical voltage. Strain gauges can be placed on the surface of the diaphragm that is not in contact with the fluid or on an element attached to the diaphragm to accomplish this. These gauges can accurately detect small diaphragm strains and provide a voltage output proportional to pressure.
This sort of transducer can accurately measure both static and dynamic pressures, as well as small and big pressures.
Strain-gage pressure transducers:
A diaphragm deflects between two chambers that are open to pressure inputs. The strain gauge expands as the diaphragm stretches in response to a change in pressure differential across it, and a Wheatstone bridge circuit amplifies the output. A capacitance transducer operates in a similar way, but instead of measuring resistance change as the diaphragm extends, it measures capacitance change.
Although strain-gage transducers can be built to have a good frequency response 1up to about 10 kHz2, they become less sensitive at higher frequencies because the diaphragm must be stiffened to produce the greater frequency response. As an alternative, a piezoelectric crystal can be employed as both the elastic element and the sensor in the diaphragm.
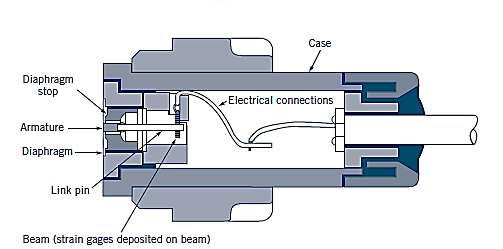
Piezoelectric transducers:
Solid-state pressure transducers work on the idea that when a crystalline substance is subjected to mechanical pressure, an electric potential is created in it. The piezoelectric (or press-electric) effect is a phenomenon first identified by brothers Pierre and Jacques Curie in 1880. Piezoelectric pressure transducers offer a far faster frequency response than diaphragm units, making them ideal for high-pressure applications. However, they are not as sensitive as diaphragm-type transducers.
The distortion of the crystal causes a voltage to arise when pressure is applied to it. The applied pressure is directly proportional to the voltage. This sort of transducer can be used to measure both extremely low and very high pressures (up to about 100,000 psi) at high frequencies, depending on the design.
Examples
Q.1. At a place where the ambient pressure is 14.5 psi, a vacuum gauge linked to a chamber reads 5.8 psi. Determine the chamber's absolute pressure.
Solution:
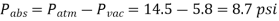
Q.2. The pressure in a tank is measured with a manometer. As demonstrated, the fluid has a specific gravity of 0.85 and the manometer column height of 55 cm. Determine the absolute pressure within the tank if the local air pressure is 96 kPa.
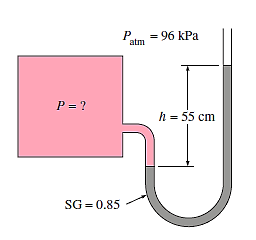
Solution:
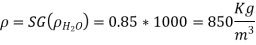
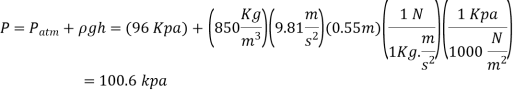
Q.3. Air pressurises the water in a tank, and the pressure is measured with a multifluid manometer, as depicted. The tank is situated on a mountain at 1400 metres above sea level, with an atmospheric pressure of 85.6 kPa. If h1 = 0.1 m, h2 = 0.2 m, and h3 = 0.35 m, calculate the air pressure in the tank. Water, oil, and mercury have densities of 1000 kg/m3, 850 kg/m3, and 13,600 kg/m3, respectively.
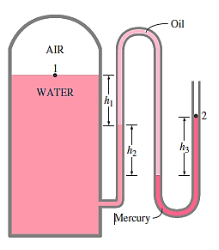
Solution:
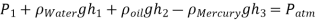
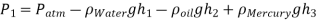
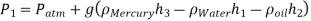

Q.4. Calculate the atmospheric pressure at a place with a barometric pressure of 740 mm Hg and a gravitational acceleration of 9.81 m/s2. Assume that mercury is at a temperature of 10°C and has a density of 13,570 kg/m3.
Solution:
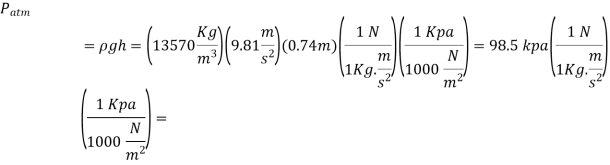
Q.5. As indicated, the piston of a vertical piston–cylinder system holding a gas weighs 60 kg and has a cross-sectional area of 0.04 m2. The gravitational acceleration is 9.81 m/s2 and the local air pressure is 0.97 bar. (a) Determine the cylinder's internal pressure. (a) Do you think the pressure inside the cylinder will change if some heat is given to the gas and its volume is doubled?
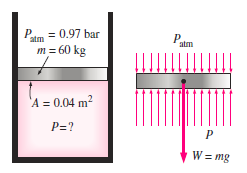
Solution:
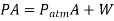

Because the volume change has no influence on the free-body diagram, the pressure inside the cylinder remains constant.
Discussion When the volume of a gas is twice under constant pressure, the absolute temperature doubles if it acts like an ideal gas.
References:
- Fluid Mechanics: Fundamentals and Applications by Y. A. Çengel and J. M. Cimbala, by McGraw-Hill
- Fundamentals of Fluid Mechanics by munson, Wiley India Pvt. Ltd
- Fluid Mechanics by Frank M. White, mcgraw Hill Publishing Company Ltd.
- Fluid Mechanics and Hydraulic Machines by R.K. Bansal, Laxmi Publications