Unit - 5
Ceramics
These are inorganic compounds that are typically formed up of metal oxides, carbides, nitrides, or silicates. Partially crystalline, partly amorphous ceramics are common. Atoms (ions) in ceramic materials act primarily as positive or negative ions and are held together by extremely strong Coulomb forces. These materials have a high compressive strength and poor ductility and are typically used as heat and electrical insulators. Glass, porcelain, and a variety of minerals are examples.
Ceramics are mixtures of metallic and non-metallic materials, with oxides, nitrides, and carbides being the most common. Aluminium oxide (or alumina, 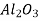 ), silicon dioxide (or silica,
), silicon dioxide (or silica, 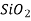 ), silicon carbide (
), silicon carbide (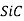 ), silicon nitride (
), silicon nitride (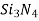 ), and, in addition, what some regard to as conventional ceramics, those made of clay minerals (i.e., porcelain), cement, and glass are all typical ceramic materials.
), and, in addition, what some regard to as conventional ceramics, those made of clay minerals (i.e., porcelain), cement, and glass are all typical ceramic materials.
Ceramic materials are relatively stiff and robust mechanically—their stiffnesses and strengths are equivalent to those of metals. Furthermore, they are usually extremely difficult. Ceramics have long been known for their exceptional brittleness (lack of elasticity) and susceptibility to fracture.
Newer ceramics, on the other hand, are being developed to have better fracture resistance; these materials are utilised in cookware, cutlery, and even vehicle engine parts. Ceramic materials are also often heat and electricity insulators, as well as being more resistant to high temperatures and severe conditions than metals and polymers. Ceramics can be transparent, translucent, or opaque in terms of optical properties, and some oxide ceramics (e.g., 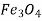 ) have magnetic properties.
) have magnetic properties.
Crystal Structure:
The crystal structures of ceramic materials with primarily ionic atomic bonding can be conceived of as being made up of electrically charged ions rather than atoms. Because they have given up their valence electrons to the non-metallic ions, or anions, which are negatively charged, metallic ions, or cations, are positively charged.
The crystal structure of crystalline ceramic materials is influenced by two features of the component ions:
- The electrical charge magnitude on each of the component ions
In terms of the first property, the crystal must be electrically neutral, which means that all of the cation positive charges must be balanced by an equal number of anion negative charges. The ratio of cations to anions, or the composition that achieves this charge balance, is indicated by a compound's chemical formula.
- The relative sizes of the cations and anions
The diameters or ionic radii of the cations 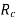 and anions
and anions 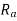 are the second requirement. Cations are often smaller than anions because metallic elements lose electrons when ionised, and hence the ratio
are the second requirement. Cations are often smaller than anions because metallic elements lose electrons when ionised, and hence the ratio
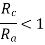
Each cation desires to have as many anions as possible in its immediate vicinity. The anions likewise want to have as many cation neighbours as possible.
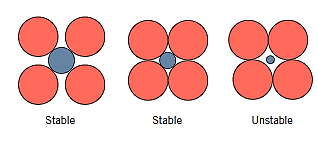
Key Takeaway:
Ceramics are mixtures of metallic and non-metallic materials, with oxides, nitrides, and carbides being the most common. Aluminium oxide (or alumina, 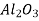 ), silicon dioxide (or silica,
), silicon dioxide (or silica, 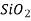 ), silicon carbide (
), silicon carbide (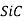 ), silicon nitride (
), silicon nitride (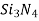 ), and, in addition, what some regard to as conventional ceramics, those made of clay minerals (i.e., porcelain), cement, and glass are all typical ceramic materials
), and, in addition, what some regard to as conventional ceramics, those made of clay minerals (i.e., porcelain), cement, and glass are all typical ceramic materials
Traditional Ceramics:
Clay, silica (quartz), and feldspar are the three main ingredients in traditional ceramics.
One of the most frequent ceramic raw materials is clay. It is frequently utilised since it is abundant in nature and is simple to manufacture. Clay is used in whitewares and structural clay items (bricks, pipes, tiles) (pottery, tableware, China, sanitaryware). Clay, which is predominantly made up of hydrated aluminium silicates, 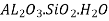 , makes up the majority of the ceramic body. Most clay products also include a low-cost filler, usually quartz, as well as feldspar, or flux, which forms a glass to link ceramic particles after heat treatment.
, makes up the majority of the ceramic body. Most clay products also include a low-cost filler, usually quartz, as well as feldspar, or flux, which forms a glass to link ceramic particles after heat treatment.
- Silicate Ceramic:
Silicates are minerals that are predominantly made of silicon and oxygen, the two most abundant elements in the earth's crust; thus, the majority of soils, rocks, clays, and sand fall into this category. Each silicon atom is bound to four oxygen atoms at each of the tetrahedron's four corners. The silicon atom is in the core of the structure. Because it is the fundamental unit of silicates, it is frequently referred to as a negatively charged substance. Because of the considerable covalent character of the interatomic Si–O bonds, which are directed and rather strong, silicates are frequently not considered ionic.
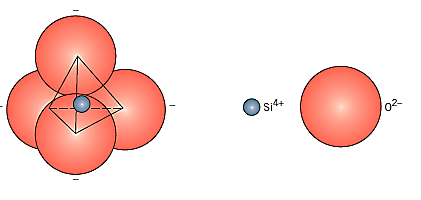
Silicon dioxide, sometimes known as silica, is the most basic silicate substance in terms of chemistry (SiO2). It's a three-dimensional network that forms when the corner oxygen atoms in each tetrahedron are shared by neighbouring tetrahedra. As a result, the material is electrically neutral, and the electronic configurations of all atoms are stable.
A crystalline structure is generated when these tetrahedra are arranged in a regular and orderly pattern. Quartz, cristobalite, and tridymite are the three major polymorphic crystalline forms of silica. Their structures are both intricate and open, meaning that the atoms are not tightly packed together. As a result, the density of these crystalline silicas is rather low.
- Clay:
Clay is one of the most used ceramic basic materials. This low-cost material, which is abundant in nature, is frequently utilised as mined without any quality enhancement. Another reason for its appeal is the ease with which clay items can be made; when clay and water are mixed in the correct quantities, they form a malleable mass that is easy to shape. After drying to remove part of the moisture, the produced component is burned at a high temperature to improve its mechanical strength.
New Ceramics:
These consist of highly purified aluminium oxide(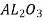 ),silicon carbide(
),silicon carbide(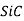 )and silicon nitride (
)and silicon nitride (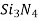 )
)
Key Takeaway:
Clay, silica (quartz), and feldspar - traditional ceramics.
Aluminium oxide(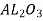 ),silicon carbide(
),silicon carbide(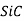 )and silicon nitride (
)and silicon nitride (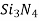 )- New ceramics
)- New ceramics
Metallic (e.g., Al, Zr, Ti, Mg) or metalloid (Si) components are combined with oxygen to form oxide ceramics. To make more sophisticated oxynitride or oxycarbide ceramics, oxides can be mixed with nitrogen or carbon. High melting points, low wear resistance, and a wide spectrum of electrical properties characterise oxide ceramics.
Non-oxide ceramics are inorganic, non-metallic ceramics used in technical applications. They have covalent bonding, can be conductive (carbides) or non-conductive (nitrides), and are commonly made up of boron, silicon, or aluminium.
- Silicon carbides having a high porosity that lower temperature and corrosion resistance while also allowing lubricants to pass through. Silicon carbides are tougher and better at conducting heat than other ceramics. In corrosive settings, they are utilised as abrasive Grinding grains (carborundum), mechanical seals, rotors in exhaust gas turbines, and heat exchangers.
- After diamond and boron nitrides, boron carbides have the highest hardness rating and offer the best resistance to abrasive wear. They're utilised to make nozzles, dressing machines, and hard metal lapping.
- Toughness and flexural strength are both high in silicon nitride ceramics. They're utilised for cutting and milling ceramics, as well as in exhaust gas turbochargers and as thermal element protection pipes.
- Aluminium nitrides can be metallized and are excellent for hard soldering. They have a high heat-conducting capability and insulating resistance. They can also be used as a printed circuit board backing.
- Boron nitrides are available in a variety of masses and are employed as hardened steel cutting tools, extrusion die plungers, forming work sprays, and nanopowders. When polymer additives and boron nitrides are combined, they produce excellent heat conductors with high electrical resistance at extremely low temperatures.
Key Takeaway:
Metallic (e.g., Al, Zr, Ti, Mg) or metalloid (Si) components are combined with oxygen to form oxide ceramics.
Non-oxide ceramics are inorganic, non-metallic ceramics used in technical applications. They have covalent bonding, can be conductive (carbides) or non-conductive (nitrides), and are commonly made up of boron, silicon, or aluminium.
Diamond:
At ambient temperature and atmospheric pressure, diamond is a metastable carbon polymorph. It has a variation of the zinc blende crystal structure, in which carbon atoms occupy all positions (both Zn and S),
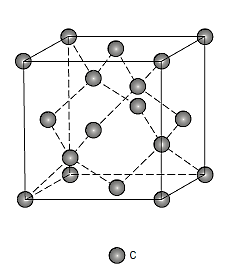
Diamond's physical qualities make it an exceptionally appealing substance.
Because of its crystal structure and strong interatomic covalent bonding, it is exceedingly hard (the hardest known material) and has a very low electrical conductivity. It also has a high index of refraction, is optically transparent in the visible and infrared parts of the electromagnetic spectrum and has an extraordinarily high heat conductivity for a non-metallic substance. As jewels, relatively big diamond single crystals are employed. Diamonds are used in industry to grind or cut softer materials.
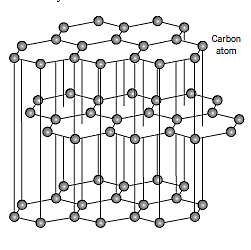
Graphite:
Graphite is a polymorph of carbon that has a crystal structure that is distinct from diamond's and is also more stable at ambient temperature and pressure than diamond. Each carbon atom is connected to three coplanar neighbour atoms by strong covalent bonds inside the graphite structure, which is made up of layers of hexagonally organised carbon atoms.
Between the layers, the fourth bonding electron engages in a weak van der Waals type of bond. Interplanar cleavage is easy as a result of the weak interplanar bonds, which provides graphite its outstanding lubricative characteristics. In addition, in crystallographic orientations parallel to the hexagonal sheets, electrical conductivity is relatively high.
High strength and chemical stability at elevated temperatures and in nonoxidizing atmospheres, great thermal conductivity, low coefficient of thermal expansion and high resistance to thermal shock, high adsorption of gases, and good machinability are all desirable qualities of graphite.
Electric furnace heating elements, arc welding electrodes, metallurgical crucibles, casting moulds for metal alloys and ceramics, high temperature refractories and insulations, rocket nozzles, chemical reactor vessels, electrical contacts, brushes, and resistors, battery electrodes, and air purification devices are all common uses for graphite.
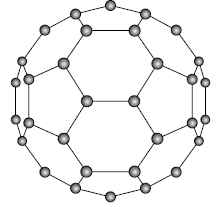
Fullerenes:
It occurs in discrete molecular form and is made up of a hollow spherical cluster of sixty carbon atoms; C60 denotes a single molecule. Each molecule is made up of bound groups of carbon atoms that form hexagonal (six carbon atom) and pentagonal (five carbon atom) geometrical shapes.
It is made up of 20 hexagons and 12 pentagons that are arranged in such a way that no two pentagons share a similar side, giving it the symmetry of a soccer ball. Buckminsterfullerene is the name given to a substance made up of C60 molecules. Fullerene is a name used to describe a class of materials made up of this sort of molecule. Diamond and graphite are what are known as network solids because all of the carbon atoms make primary bonds with neighbouring atoms throughout the solid.
This substance is electrically insulating since it is a pure crystalline solid. It can, however, be made extremely conductive and semiconductive with the right impurity additions.
Key Takeaway:
Carbon (sometimes also considered a ceramic) may exist in several polymorphic forms:
- Diamond
- Graphite
- The fullerenes
Each of these materials has its own unique properties:
Diamond—hardness, high thermal conductivity
Graphite—high-temperature chemical stability, good lubricative properties
Fullerenes—electrically insulative, conductive, or semiconductive
Carbon nanotubes—extremely strong and stiff, electrically conductive or semiconductive
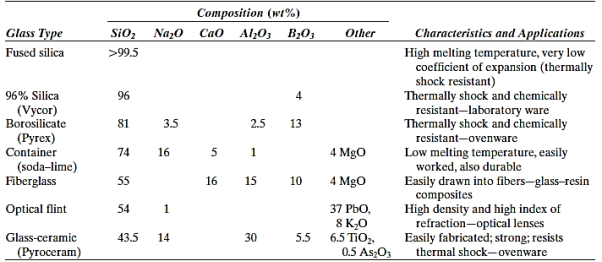
Containers, lenses, and fibreglass are common applications for the glasses, which are a recognised group of ceramics. They are non-crystalline silicates including additional oxides, such as CaO, Na2O, K2O, and Al2O3, which influence the glass characteristics, as previously stated. A typical soda–lime glass contains roughly 70% SiO2, with the rest being mostly Na2O (soda) and CaO. (lime).
Key Takeaway:
- The familiar glass materials are non-crystalline silicates that contain other oxides. In addition to silica (SiO2), the two other primary ingredients of a typical soda–lime glass are soda (Na2O) and lime (CaO).
- The two prime assets of glass materials are optical transparency and ease of fabrication.
Glass ceramics:
The following characteristics of glass-ceramic materials have been designed: relatively high mechanical strengths; low coefficients of thermal expansion (to avoid thermal shock); relatively high temperature capabilities; good dielectric properties (for electronic packaging applications); and good biological compatibility. Optical transparency can be achieved with some glass-ceramics, while opaqueness is achieved with others. The ease with which this class of materials can be made is perhaps its most appealing feature; ordinary glass-forming procedures can be utilised to mass-produce practically pore-free ware with ease.
Ovenware, tableware, oven windows, and range tops are the most typical applications for these materials, owing to their robustness and good thermal shock resistance. They're also utilised for architectural cladding, heat exchangers, and regenerators, as well as electrical insulators and substrates for printed circuit boards.
Refractories:
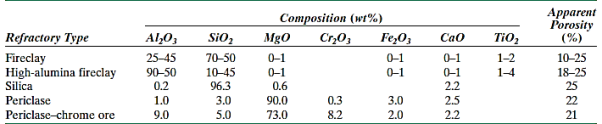
Refractory ceramics are another important kind of ceramics used in high tonnages. The ability to tolerate high temperatures without melting or decomposing, as well as the ability to stay unreactive and inert when exposed to harsh environments, are two of these materials' most important characteristics. Furthermore, the ability to provide thermal insulation is frequently a factor to consider. Refractory materials come in a range of shapes and sizes, although bricks are the most popular. Furnace linings for metal refining, glass manufacture, metallurgical heat treatment, and power generation are examples of typical applications.
The raw ingredients for many commercial materials include both large (or grog) and tiny particles, which may have distinct compositions. The small particles are generally involved in the creation of a bonding phase during firing, which is responsible for the brick's improved strength; this phase might be primarily glassy or crystalline. The temperature at which the refractory component is used is usually lower than the temperature at which it was burned.
One microstructural variable that must be regulated in order to make a proper refractory brick is porosity. Porosity reduction increases strength, load-bearing capability, and resistance to corrosive materials attack. At the same time, thermal insulating properties and thermal shock resistance deteriorate. Of course, the ideal porosity is determined by the service conditions.
Abrasives:
Abrasive ceramics are used to wear, grind, or cut away other, typically softer material. As a result, hardness or wear resistance is the most important requirement for this group of materials; also, a high degree of toughness is required to ensure that the abrasive particles do not easily shatter. Furthermore, because abrasive frictional forces can cause high temperatures, certain refractoriness is desirable.
Abrasives made of natural and synthetic diamonds are used as abrasives, however they are rather expensive. Silicon carbide, tungsten carbide (WC), aluminium oxide (or corundum), and silica sand are the most popular ceramic abrasives.
Abrasives come in a variety of forms, including bonded to grinding wheels, coated abrasives, and loose grains. The abrasive particles are bound to a wheel using a glassy ceramic or an organic resin in the first scenario. The surface structure should have some porosity; excessive heating is prevented by a continuous flow of air currents or liquid coolants within the pores that surround the refractory grains.
Sandpaper is probably the most well-known example of coated abrasives, in which an abrasive powder is coated on some form of paper or cloth material. This type of abrasive is commonly used to grind and polish wood, metals, ceramics, and polymers.
Loose abrasive granules carried in an oil or water-based carrier are commonly used in grinding, lapping, and polishing wheels. In loose form, diamonds, corundum, silicon carbide, and rouge (an iron oxide) are utilised in a variety of grain sizes.
Cements:
Inorganic cements include a variety of well-known ceramic materials such as cement, plaster of Paris, and lime, all of which are mass-produced in vast amounts. These materials have the distinct property of forming a paste when mixed with water, which then sets and hardens. This property is very valuable since it allows solid and rigid structures of almost any shape to be quickly created.
Some of these materials also serve as a bonding phase, chemically binding particle aggregates into a single, cohesive structure. The cement's role is comparable to that of the glassy bonding phase that occurs when clay products and some refractory bricks are burnt under these conditions. However, one significant difference is that the cementitious bond forms at ambient temperature.
The most tonnages of this group of materials are consumed by portland cement. It is made by grinding and thoroughly mixing clay and lime-bearing minerals in the right quantities, then heating the mixture in a rotary kiln to around 1400C (2550F); this process, known as calcination, causes physical and chemical changes in the raw materials.
Because its hardness develops through chemical reactions with water, Portland cement is referred to as a hydraulic cement. It is primarily used to bind aggregates of inert particles (sand and/or gravel) into a cohesive mass in mortar and concrete; these are called composite materials. Nonhydraulic cements, such as lime, are those in which chemicals other than water (e.g., CO2) are involved in the hardening reaction.
Microelectromechanical Systems (MEMS):
Microelectromechanical systems (abbreviated MEMS) are miniature "smart" systems made up of a variety of mechanical devices linked to a large number of electrical elements on a silicon substrate. Microsensors and micro actuators are the mechanical components. Microsensors measure mechanical, thermal, chemical, optical, and/or magnetic phenomena to gather information about the environment.
The microelectronic components then process the sensory data and make judgments that guide reactions from the micro actuator devices, which include devices that execute tasks including positioning, moving, pumping, regulating, and filtering. Beams, pits, gears, motors, and membranes are examples of actuating devices with microscopic dimensions on the order of microns.
Optical Fibres:
The optical fibre is a novel and advanced ceramic material that is a crucial component in our current optical communications systems. The optical fibre is comprised of ultra-pure silica that must be devoid of even the tiniest impurities and other flaws that might absorb, scatter, and attenuate a light beam. To create fibres that match the stringent requirements of this application, very complex and sophisticated processing processes have been devised.
Key Takeaway:
- Glass-ceramics are made as glasses and then crystallised into fine-grained polycrystalline materials through heat treatment.
- Improved mechanical strengths and lower coefficients of thermal expansion (which increases thermal shock resistance) are two features of glass-ceramics that set them apart from glass.
- Clay is the primary ingredient in whitewares (such as pottery and dinnerware) as well as structural clay goods (e.g., building bricks and tiles). Ingredients such as feldspar and quartz, in addition to clay, may be added to impact the changes that occur during fire.
- Refractory ceramics are materials that are used at high temperatures and in often reactive settings.
- The four main subdivisions of this class of materials are fireclay (alumina–silica mixtures), silica (high silica contents), basic (rich in magnesia, MgO), and special. • The four main subdivisions of this class of materials are fireclay (alumina–silica mixtures), silica (high silica contents), basic (rich in magnesia, MgO), and special.
- Abrasive ceramics are used to cut, grind, and polish softer materials; they must be strong and durable, and they must be able to tolerate high temperatures caused by frictional forces.
- The most common abrasive materials are diamond, silicon carbide, tungsten carbide, corundum, and silica sand.
- • Portland cement is made in a rotary kiln by heating a mixture of clay and lime-bearing materials. The resulting "clinker" is ground into extremely fine particles before being mixed with a little amount of gypsum.
- When inorganic cements are combined with water, they generate a paste that can take on almost any shape.
- At room temperature, subsequent setting or hardening happens as a result of chemical processes involving the cement particles. Hydration is the chemical reaction that occurs in hydraulic cements, the most common of which being portland cement.
References:
- Material Science and Engineering by V. Raghavan
- Material Science and Engineering by William D. Callister
- Material Science by Prof. Satish V. Kailas (Associate Professor, Dept. Of Mechanical Engineering, Indian Institute of Science, Bangalore – 560012) NPTEL