Unit 05
Stereochemistry
Isomerism:
The compound having same molecular formula but different physical and chemical properties due to the different arrangement of atoms or molecules are known as isomers and the whole phenomena is called as Isomerisation.
Structural Isomerism:
It is due to the difference in ways in which different atoms or groups are linked to one another within the molecule. In this type of isomerism the isomers have different structure without taking into consideration the arrangement of groups in space. These are further classified as:
Chain Isomerism:
It arises due to the different arrangement of C atoms that is straight or branch chain of C atoms. E.g.: n-butane &iso-butane have same molecular formula C4H10.
n-butane - straight chain
iso-butane - branched chain
Position Isomerism:
It is due to the difference in position taken up by substituent atoms or groups in same C chain or due to different position of double or triple bond. E.g.: C3H7Cl shows 2 isomeric compound i.e.; n-propyl chloride and iso-propyl chloride.
Ring Chain Isomerism:
This type of isomerism arises due to the difference in type of linking of C-atom & isomer may have either open chain or closed chain structure.
Functional Isomerism:
It arises due to the difference in nature of functional group present in isomeric compound. E.g.: C2H6O
CH3-O-CH3 CH3-CH2-OH
Dimethyl ether ethanol
Stereoisomerism:
Compounds having identical structure but difference in their configuration i.e.; relative arrangement of atoms or groups within the molecule or different in space are called stereoisomers. The phenomena is called as the stereoisomerism. These compound are said to have different configuration.
Configurations:
The representation of the atoms and molecules with respect to each other in organic molecule is called as the configurations.
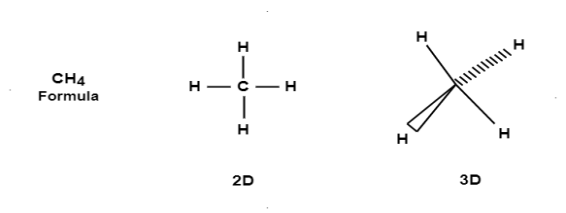
E.g. Consider the methane molecule with molecular formula of CH4. It’s 2D & 3D configuration are
|
Chirality:
Chirality is a useful concept in physical and life sciences, especially when applied to a molecular level. It derives from the Greek work their meaning “hand” because, like hands, the molecules and objects in general are not super imposable with their mirror images. The term chiral was coined by Lord Kelvin in his Baltimore lectures, although its usage remained ignored for nearly one century, being rediscovered by the mid-1960s to define a geometrical model devoid of certain symmetry elements, with the exception of an axis of rotation. Chirality manifests itself in both molecules and crystals, and its origin lies clearly in molecular architecture. The analogy between crystals and molecules in this context was first caught by Pasteur, who realized that the nonidentity of the crystal (or molecule) with its mirror image was due to what he called dissymmetry (we would now say chirality). Although Pasteur himself conjectured on different structural arrangements, including tetrahedral, he was unaware of the tetrahedral asymmetric carbon postulated by van't Hoff and Le Bel in 1874. The two forms of a chiral molecule are called enantiomers and have identical physical and chemical properties, although they will rotate the plane of polarized light in opposite senses (i.e., optical activity). Two enantiomers will usually differ in reactivity in the presence of other chiral molecules, a fact of enormous significance in biology and drug discovery. Chiral and related words (e.g., homochiral, heterochiral) have engendered both confusion and ambiguity in the literature and, unfortunately, improper usage is not unusual. A brief, yet convenient, discussion illustrates the points. A single chiral molecule will either be right-handed or left-handed. The term chiral is insufficient, however, when applied to a compound or sample (i.e., a macroscopic collection of molecules), because it does not imply that we are dealing with molecules having the same sense of chirality. Introduction of homochiral or heterochiral labels are appropriate to this end. Moreover, homochiral means that the sample is made up of molecules with the same sense of chirality or handedness, which does not necessarily imply the existence of a single enantiomer. Clearly, one needs to know if the sample in question is actually racemic, nonracemic, in which the chiral sample contains a certain excess of the major enantiomer. Finally, as one would have expected for a term restricted to molecules and objects, chiral and chirality should not be employed for dynamic transformations (e.g., chiral synthesis, chiral separation, chiral amplification and so on) despite their widespread use. The situation may even be more complex in crystals because a racemic crystal is not equivalent to a racemic compound in terms of molecular composition. In addition, the distinctive nature of stereoisomers in the solid state is crucial to understand the separation and resolution of enantiomers by crystallization.
Lewis Pastues while studying crystallography of salt of tartaric acid. He observed that optical inactive Na, NH3, tartrate exists as mixture of 2 different type of crystals which mirror images of each other. These crystal which gives the mirror images of each other are called enantiomorphs& the phenomena are called as the enantiomorphism. Although the original mixture was optically inactive each type of crystal when dissolved in water were found to be optically active. The specific rotation of 2 solution were exactly equal but of opposite sign i.e.; 1 solution rotated the PPL to the right or clockwise while other to the left or anti clockwise to the same extent, 2 type of crystal or solution were identical in all other physical and chemical properties. Isomers which are non super imposable mirror images of each other are called enantiomers.
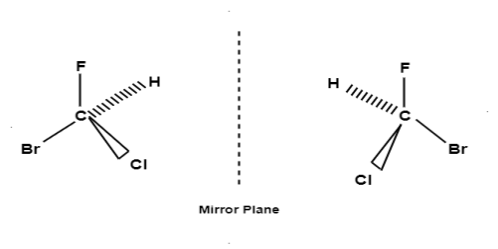
According to the Le-Bell and Van’t Hoff the 4 valencies of C atom are directed towards the 4 corners of a regular tetrahedron at the centre of which C atom lies. Consider a compound CLMNO in which L,M,N,O are 4 different group or atom attached to the C atom may be represented by 2 models:
|
It is important to know that these 2 molecules can’t be super imposed on each other.
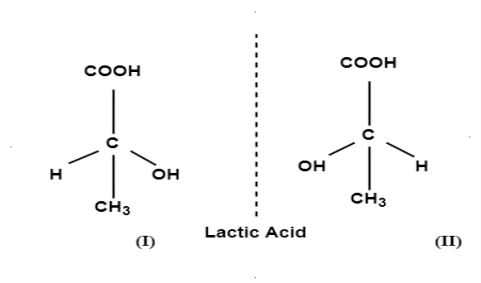
E.g.; Lactic acid and secondary butyl chloride exists as 2 optically active isomers which are enantiomers i.e.; mirror images of each other. Mirror images of 2 compounds are shown as:
|
Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are nonsuper-imposable, non-mirror images.
Enantiomers and diastereomers commonly called stereoisomer’s fall under the broader concept of isomerism which always involves the comparison of at least two species.Stereoisomers are molecules of identical constitution but nevertheless different. Differences between diastereomers can be expressed in scalar terms, that is by differences in the distances of certain characteristic pairs of atoms. Compounds having identical structure but difference in their configuration i.e.; relative arrangement of atoms or groups within the molecule or different in space are called stereoisomers. The phenomena is called as the stereoisomerism. These compounds are said to have different configuration.
Certain substance have strange behavior. When a PPL is passed through the solution of such substance the light coming out of the solution is found to be on different plane. The PPL is rotated. The angle of rotation of PPL is known as the optical rotation. The substance which rotate the PPL to clockwise direction or right direction are dexo-rotatory while the vice versa is called as the levo rotatory. Substance which do not rotate the PPL are said to be optically inactive.
The instrument used for measuring optical rotation is called polarimeter. It consists of light source to nicol prism and in between a tube to hold solution of organic substance. The schematic representation of polarimeter is given as –
|
Absolute configurations ( R and S Configuration with Ex.)
Carbon, when neutral, is bonded to four groups in a tetrahedral arrangement. What this means is that each group points as far away from the other in three-dimensional space as it can. Imagine carbon in the center of the tetrahedron shown below, with each of the four atoms that it is bonded to pointing in the direction of each corner. This describes the tetrahedral geometry of carbon. When a molecule contains a chiral carbon atom, there are four different groups attached to the carbon. Because of the way the groups orient themselves in space, the molecule can exist in one of two configurations at that carbon, called the absolute configuration. If the molecule has just one chiral carbon, these two configurations are called enantiomers. The two possible molecules are mirror images of each other, but they are non-superimposable. Take a peek at the two organic molecules below; they are mirror images, but if you tried to lay them on top of each other, the groups would never line up perfectly. This is what is meant by 'non-superimposable. This is a tough concept, so let's consider a real life example. Take the palms of your hands and face them toward each other. The thumbs line up, and so do your pinky fingers. The hands are mirror images. Now, try to put one hand on top of the other with he palms facing down. No matter how we position the hands, the thumbs will never line up. This is because they are non-superimposable.
|
You are more chiral than just your hands. In fact, 19 out of 20 of the amino acids that make up your body are chiral. The proteins that carry out your biological functions are made of these amino acids, so your proteins are chiral too. This means that they can interact with molecules containing chiral carbons distinctly. A protein may recognize one enantiomer of an organic molecule as a drug, and the enantiomer of that same organic molecule may have little to no effect.
The spatial arrangement of the atoms of a chiral molecular group and its stereo chemical description.
R means Rectus while the S stands for the Sinister in regular term Rectus is orientation of molecule in clock wise direction while Sinister means the orientation of the molecule in anti clockwise direction. R-S configuration is used to give the identity to the elements attached to the chiral atoms.
The molecule must be arranged according to the priority order.
Priority order depends on the atomic number of the molecule. (the greater atomic number the greater is priority order and vice versa)
|
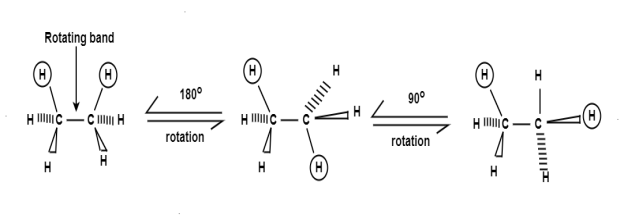
Conformational analysis(Staggered and eclipsed Conformation of Ethane):
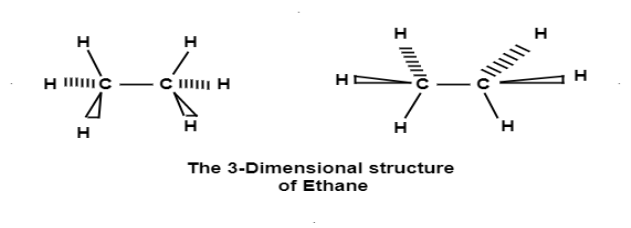
The 3 dimensional structure of ethane(C2H6):
|
Ethane Confirmations:
Ethane molecule consists of 7 sigma bond on the rotation there are no changes noticed in the 6 carbon hydrogen molecule as because the hydrogen are essentially spherical.
|
Ethane Configuration
In order to better visualize these different conformations, it is convenient to use a drawing convention called the Newmanprojection. In a Newman projection, we look lengthwise down a specific bond of interest – in this case, the carbon-carbon bond in ethane.
|
The hydrogen and carbon atom depicted above shows the 120obond angles. While the lowest conformation of ethane is called as the Staggered Conformationin which all of the C-H bonds on the front carbon are positioned at dihedral angles of 60°relative to the C-H bonds on the back carbon. In this conformation, the distance between the bonds (and the electrons in them) is maximized.
|
The preparation of organic compound from the commercial chemicals.Chemical synthesis can also be used to prove the chemical structure of a compound. The structure of a compound isolated from natural sources was deduced by chemical reactions that converted the original compound into substances of known, smaller molecular arrangements.
In 1979, chemical synthesis was used as a tool to determine the molecular structure of periplanone B. C. J. Persons isolated 200 micrograms of periplanone B from the droppings of 75,000 virgin female cockroaches. He was able to deduce the gross chemical structure of the compound by modern analytical methods, but not its exact three dimensional structure. Without knowing the stereochemistry or three dimensional arrangement of the carbonatoms, larger quantities of the excitant could not be prepared and tested. In 1979, W. Clark Still at Columbia University in New York, set out to determine the structure of periplanone B. He noted that four compounds had to be made by chemical synthesis in order to determine the structure of the cockroach excitant. He chose an easily prepared starting material and by a series of chemical reactions was able to make three of the four compounds he needed to determine the chemical structure. One of the new substances matched all the analytical data from the natural material.Organic chemistry deals with the organic compounds that contain C, H, O. These are chemical reaction that involves organic compounds.
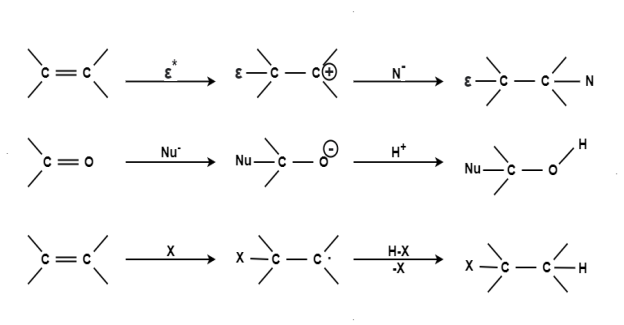
Substitution Reaction: When a reactant A-B reacts; C comes out as the leaving group i.e.; it has taken a place of B or in other words it has substitution reaction. In other words a substitution reaction is a part of one molecular replaced by other atom or group without causing a change a change in rest of the molecule. E.g. A-B + C A-C + B CH4 + Cl2 CH3Cl + HCl CH3Cl + Cl2 CH2Cl2 + HCl CH2Cl2 + Cl2 CHCl3 + HCl CHCl3 + Cl2 CCl4 + HCl The substitution reaction may be brought by free radical nucleophilic or electrophilic reagent. Mechanism: The substitution reaction may be completed through following steps: Chain Initiation: The formation of chlorine free radical took place by the presence of sunlight or ultraviolet rays. Cl : Cl Clo + Clo
Chain Propagation: The Cl2 free radical replaces H2 atom forming an other free radical which can later again formed Cl2 free radical & thus this chain goes on till all the H2 atoms are reduced by the chlorine. Clo + H:CH3 HCl + oCH3 oCH3 + Cl:Cl CH3Cl + oCl oCl + H:CH2Cl HCl + oCH2Cl oCH2Cl + Cl:Cl CH2Cl2 + oCl oCl + H:CH2Cl2 HCl + oCHCL2 oCHCL2 + Cl:Cl CHCl3 + oCl oCl + H:CCl3 HCl + oCCl3 oCCl3 + Cl:Cl CCl4 + oCl Chain Termination: When all hydrogen atom are replaced by Cl than finally 2Cl free radical combines & chains terminates to form Cl molecule. Clo + oCl Cl:Cl
Addition Reaction:Addition reaction is one of the simplest term in the organic chemistry this involve the expansion of the chain by addition of different molecule.
|
Elimination Reaction:
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Mechanism
The elimination reaction consists of three fundamental eventsthey are:
- C-C pi bond is formed.
- Proton removal.
- There is a breakage in the bond of the leaving group.
Depending on the reaction kinetics, elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the molecularity.
E1 Reaction
- This is also called as unimolecular elimination reaction, thereare usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
|
E2 Reaction:
- In an E2 mechanism which refers to bimolecularelimination is basically a one-step mechanism.
- Here, the carbon-hydrogen and carbon-halogen bonds mostly break off to form a new double bond.
- However, in the E2 mechanism, a base is part of the rate-determining step and it has a huge influence on the mechanism.
- The reaction rate is mostly proportional to the concentrations of both the eliminating agent and the substrate.
- It exhibits second-orderkinetics.
The E2 mechanism can generally be represented as below. In the below-mentioned representation, B stands for base and X stands for the halogen.
|
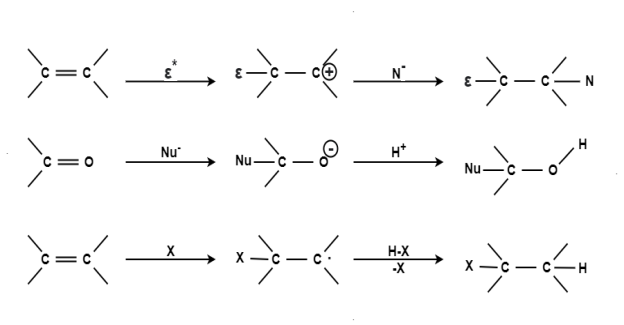
Substitution Reaction: When a reactant A-B reacts; C comes out as the leaving group i.e.; it has taken a place of B or in other words it has substitution reaction. In other words a substitution reaction is a part of one molecular replaced by other atom or group without causing a change a change in rest of the molecule. E.g. A-B + C A-C + B CH4 + Cl2 CH3Cl + HCl CH3Cl + Cl2 CH2Cl2 + HCl CH2Cl2 + Cl2 CHCl3 + HCl CHCl3 + Cl2 CCl4 + HCl The substitution reaction may be brought by free radical nucleophilic or electrophilic reagent. Mechanism: The substitution reaction may be completed through following steps: Chain Initiation: The formation of chlorine free radical took place by the presence of sunlight or ultraviolet rays. Cl : Cl Clo + Clo
Chain Propagation: The Cl2 free radical replaces H2 atom forming an other free radical which can later again formed Cl2 free radical & thus this chain goes on till all the H2 atoms are reduced by the chlorine. Clo + H:CH3 HCl + oCH3 oCH3 + Cl:Cl CH3Cl + oCl oCl + H:CH2Cl HCl + oCH2Cl oCH2Cl + Cl:Cl CH2Cl2 + oCl oCl + H:CH2Cl2 HCl + oCHCL2 oCHCL2 + Cl:Cl CHCl3 + oCl oCl + H:CCl3 HCl + oCCl3 oCCl3 + Cl:Cl CCl4 + oCl
Chain Termination: When all hydrogen atom are replaced by Cl than finally 2Cl free radical combines & chains terminates to form Cl molecule. Clo + oCl Cl:Cl
Addition Reaction:Addition reaction is one of the simplest term in the organic chemistry this involve the expansion of the chain by addition of different molecule.
|
Elimination Reaction:
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Mechanism
The elimination reaction consists of three fundamental eventsthey are:
4. C-C pi bond is formed.
5. Proton removal.
6. There is a breakage in the bond of the leaving group.
Depending on the reaction kinetics, elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the molecularity.
E1 Reaction
- This is also called as unimolecular elimination reaction, thereare usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
|
E2 Reaction:
- In an E2 mechanism which refers to bimolecularelimination is basically a one-step mechanism.
- Here, the carbon-hydrogen and carbon-halogen bonds mostly break off to form a new double bond.
- However, in the E2 mechanism, a base is part of the rate-determining step and it has a huge influence on the mechanism.
- The reaction rate is mostly proportional to the concentrations of both the eliminating agent and the substrate.
- It exhibits second-orderkinetics.
The E2 mechanism can generally be represented as below. In the below-mentioned representation, B stands for base and X stands for the halogen.
|
Oxidation-Reduction Reaction:
Redox reactions are comprised of two parts, a reduced half and an oxidized half, that always occur together. The reduced half gains electrons and the oxidation number decreases, while the oxidized half loses electrons and the oxidation number increases. Simple ways to remember this include the mnemonic devices OIL RIG, meaning oxidation is loss and reduction is gain, and LEO says GER, meaning loss of e- = oxidation and gain of e- = reduced. There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken up by another species in the reduction half reaction.
The two species that exchange electrons in a redox reaction are given special names. The ion or molecule that accepts electrons is called the oxidizing agent; by accepting electrons it causes the oxidation of another species. Conversely, the species that donates electrons is called the reducing agent; when the reaction occurs, it reduces the other species. In other words, what is oxidized is the reducing agent and what is reduced is the oxidizing agent.
A good example of a redox reaction is the thermite reaction, in which iron atoms in ferric oxide lose (or give up) O atoms to Al atoms, producing Al2O3.
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(l)
|
Aspirin:
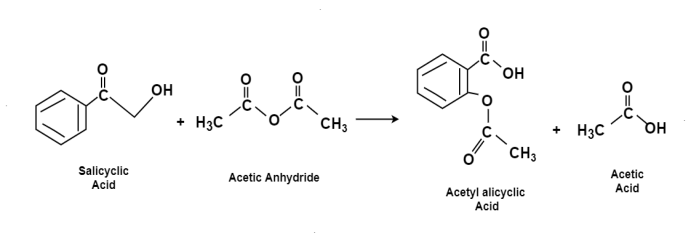
The chemical name for the Aspirin is Acetylsalicyclic acid. It is used as pain killer and fever reducer. Salicyclic acid derive from the willow family of plants which were widely used for treating headache. For the preparation of aspirin the salicyclic acid is reacted with the excess acetic anhydride.Phospohoric acid is used for boosting the procedure of reaction. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. The synthesis reaction of aspirin is shown below:
|
Since acetic acid is very soluble in water, it is easily separated from the aspirin product. The aspirin isolated in this step is the “crude product”. A “purified product” can be obtained through re-crystallization of the crude product in hot ethanol. In this experiment, the crude product will be the desired product. The percent yield of the crude product will be determined for this reaction. The purity of the product will also be analyzed. The product will be analyzed by three different methods: melting point, titration, and spectroscopic assay. C and the melting point range of the salicylic acid. The melting point range of pure aspirin is 138-140 C. If impurities are present in your crude sample, the melting point rangestarting material is 158-161 for your product will be lower than the range of pure aspirin. Also, your melting point range may be greater than 2 degrees.
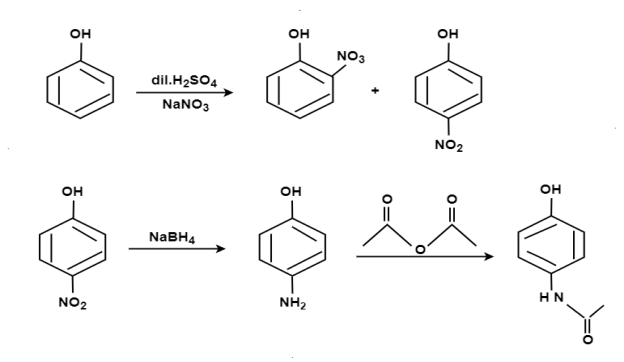
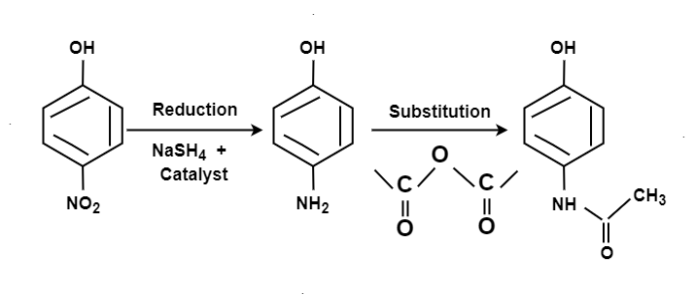
Paracetamol: From Phenol:
From Para nitro Phenol
|
Reference Books
1. B. H. Mahan University chemistry, Pearsons Publication, 4th edition
2. M. J. Sienko and R. A. Plane, Chemistry: Principles and Applications,
3. C. N. Banwell, Fundamentals of Molecular Spectroscopy,Mcgraw Higher Ed., 4th edition.
4.P. W. Atkins, Physical Chemistry, Oxford University Press, 7th edition.
5. J. D. Lee Concise Inorganic Chemistry ,Oxford University Press, 5 th edition
6. Puri,Sharma, Kalia, Principles of Inorganic Chemistry