UNIT-3
Engineering Materials
Monomer and its functionality
Monomer, is a molecule that consists of any class of compounds that are mostly organic, and react with other molecules to form very large molecules or polymers. The essential feature of monomer is its polyfunctionality, that is they are capable of forming chemical bonds with two other monomer molecules. Monomers are also functional and can form only linear, chainlike polymers. Cross linked network polymer products are formed from higher functionality polymers.
Addition reactions that consist of double bonds between two atoms or ring from three to seven atoms are characteristic of monomers; examples include styrene, caprolactam (which forms nylon-6), and butadiene and acrylonitrile (which copolymerize to form nitrile rubber, or Buna N)
Polymers and degree of polymerization
A polymer is a large molecule with high molecular weight, this high molecular weight is obtained when smaller molecules having low molecular weight of one or more types undergo the chemical interaction. The process of manufacture of a polymer is known as polymerization.
Polymer, either they are natural or synthetic substances consists of very large molecules called macromolecules, these macromolecules are units or multiples of simpler chemical units called monomers. Polymers find their space in many of the materials in living organisms, including, for example, proteins, cellulose and also nucleic acids. They are also present in manmade materials such as concrete, glass, plastics and rubbers, polymers also constitute the basis of such minerals like diamond, quartz and feldspar.
The word polymer designates an unspecified number of monomer units. When the number of monomers is very large, the compound is sometimes called a high polymer
The degree of polymerization (DP or Xn) is defined as the number of monomer units in the polymer. It is calculated as the ratio of molecular weight of a polymer and molecular weight of the repeat unit. Number average DP and weight average DP are the two main types used for measuring the DP.
Classification of Polymers
One important classification of plastics is by the characteristic of permanence or impermanence of their form, or whether they are: thermoplastics or thermosetting polymer.
Thermoplastics these are plastics that do not show any changes in their chemical composition when subjected to heating, therefore they can be moulded again and again. Examples include: PVC, polystyrene and polypropylene.
Thermosets, or thermosetting polymers, when heated will melt and will take shape only once it cannot be moulded any further once they are solidified. If they are heated again, they do not melt but decompose. An irreversible chemical reaction occurs in the thermosetting process. A good example of thermosetting polymers is vulcanization of rubber is an example of a thermosetting process.
Types of polymerization
Addition polymerization
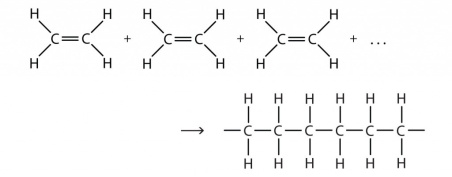
In Addition, polymerization, molecules of the same or different monomers add up together to form a polymer on a large scale. When monomers are being added to form a large chain such a process is known as chain growth polymerization. The monomers that are used in this type of polymerization are unsaturated compounds (the compounds of carbon are connected by double or triple covalent bonds).
- For example, alkenes, alkynes and alkadienes.
The chain length is increased either by the formation of ion species or free radicals, in this case however the reaction takes place in the presence of free radicals, the mechanism is discussed below
1.Free radical mechanism:
Free generating initiator are the catalysts that polymerize the reaction of the compound of alkenes and their derivatives.
- For example, benzoyl peroxides.
When ethene is polymerised to polythene, the mixture of ethene is first heated with a small amount of the initiator benzoyl peroxide. A new and large free radicle is formed, when there is an additional free radicle formed by peroxide to the double bond of ethane. This step is known as the chain initiating step; this means that the chain formation is initiated in this very step. As the process proceeds, a bigger sized radical is formed as the free radical obtained from the first step reacts with another molecule of ethane. A chain propagating step is formed as the reaction continuous with the sequence being repeated leading to the formation of bigger and new radicals. The last stage being the chain termination step, where the radical formed and the product radical react with another radical to form the final polymerized product, and hence this is called the chain terminating step.
2. Condensation polymerization:
This type of polymerization involves a repeated condensation process between bi-functional monomers. Simple molecules such as water and alcohol are lost due to the condensation process. The Mechanism of Condensation mechanism refers to combining of smaller molecules to get larger molecules. The product that is formed in this polymerization is a bi-functional species, these species will further undergo a sequence of processes, however at each step a very unique and distinct functional species and each species that is forms is independent when compared to the other species. Hence this process is called as step growth polymerization.
- Examples, include ethylene glycol and terephthalic acid.
|
Fig 1: Polymerization Reaction
3.Copolymerization
A copolymer is a polymer that is formed from more than one species of monomers. The process of polymerization of monomers into copolymers is called as copolymerization. Biopolymers are those that are formed from copolymerization of two monomer species, subsequently those formed from three or four monomers are called terpolymers and quaterpolymers, respectively.
A copolymer consists of at least two types of structural units, based on the arrangement of these units along the chain they are divided into:
Linear Polymers: They consist of a main chain that is single that alternates with statistical and block copolymers.
Branched copolymers consist of a single main chain and consists of one or more polymeric side chains, that can be grafted, star shaped or have other architectures.
Plastics
Preparation property and uses of PVC
The physical properties include
- They are brittle and solid in nature and insoluble in Alcohol, but soluble in Hydrofluran,
- They show high amount of Hardness and show thermoplasticity, as the mechanical properties increase with the Increase in temperature.
- Heat Stability of PVC is quite poor as they begin to decompose once the temperature reaches 140 °C, the melting point of PVC is 160 °C.
- The elasticity of PVC reaches to 1500-3000 Mpa. They have ordinary friction.
- PVC shows good insulation properties, but its insulating property is less than that of Polyethylene.
- As the dielectric constant, dielectric loss tangent value and volume resistivity are high, the corona resistance is not very good, it is generally suitable for medium or low voltage and low frequency insulation materials
Chemical structure:
H Cl | | – C – C – | | H H
|
PREPARATION:
poly vinyl chloride is prepared by polymerisation of monomer vinyl chloride
– About 80 percent of polymerisation includes suspension polymerisation.
-12% emulsion polymerisation and 8% bulk polymerisation.
Process: In goes the VCM and water are into the reactor and a polymerization initiator, along with other additives. The pressure in the vessel is kept tight to keep the VCM. To ensure a uniform particle size of the PVC resin, they are continuously mixed. The reaction is exothermic, and thus requires cooling. The suspension needs to be in its form therefore water is added as PVC is denser than VCM.
The polymerization of VCM is started by the droplets being mixed by compounds called initiators, the radical chain reaction begins when these initiators break down. Some examples of initiators include dioctanoyl peroxide and diacetyl peroxydicarbonate, both of which have fragile O-O bonds .to bring about a uniform rate to a polymerization reaction, a combination of initiators is used, as some initiators start the reaction rapidly but later slow down while others show the opposite therefore a combination of initiators are used to bring a steady rate to polymerization reaction. One the polymer grows to a size of 10x, the short polymers that are formed precipitate inside the droplet of VCM, and the process continues with the precipitated particles that are solvent swollen in nature.
On the course of the reaction, a PVC slurry is formed which is subjected to degas and stripping to remove excess VCM which is also recycled. Later the polymer is then passed through a centrifuge to remove water. The slurry is further dried in a hot air bed, and the resulting powder sieved before storage or pelletization. Normally, the resulting PVC has a VCM content of less than 1 part per million.
Uses:
PVC in its hard form is used in:
- Machined Parts
- Chemical Resistant Applications
- Wet Benches
- Vinyl records
- Plumbing and electrical fixtures
Bakelite
Bakelite is the commercial name for the polymer obtained by the polymerization of phenol and formaldehyde. These are one of the oldest polymers that were synthesized by man. Phenol is made to react with formaldehyde. The condensation reaction of the two reactants in a controlled acidic or basic medium result in the formation of ortho and para hydroxymethyl phenols and their derivatives.
Preparation and Properties of Bakelite
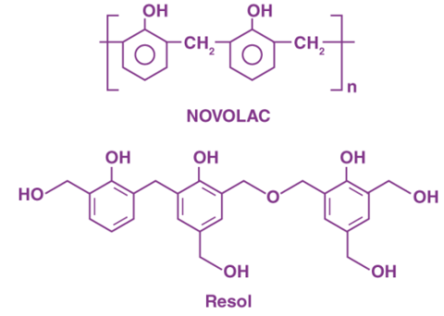
In a reaction when Phenol is present in excess, the reaction becomes acidic making the end product also acidic, however, when formaldehyde is added in more quantity than phenol the reaction becomes basic and the end product becomes basic in nature and is known as Resol.
|
Fig 2: Structures of Resol and Novolac
There are intermediate products formed during the process of condensation and these are used as resins in the industries These intermediate condensation products are used as resins in different industries. When the compound Novolac undergoes cross linking with the help of phenol, which acts as a cross linking agent. Bakelite is produced.
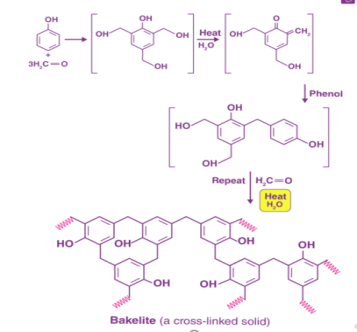
The preparation of Bakelite involves several steps, as illustrated below.
|
Fig 3: Preparation of Bakelite.
Initial methods of preparing Bakelite involved the heating of formaldehyde and phenol in the presence of one of the following catalysts -zinc chloride (ZnCl2), hydrochloric acid (HCl), or ammonia (NH3).
Bakelite Structure
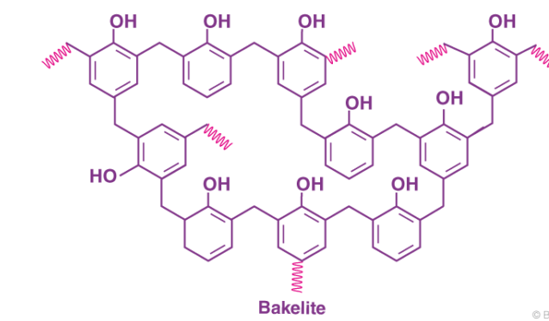
The cross-linked product of phenol and formaldehyde has the following structure.
|
Fig 4: The chemical formula of Bakelite can be written as (C6H6O-CH2OH) n.
Properties of Bakelite
Some important properties of Bakelite are listed below.
- Bakelite can be quickly moulded.
- The smooth mouldings can be obtained with this polymer.
- Bakelite mouldings are heat-resistant and scratch-resistant.
- Bakelite is also resistant to several destructive solvents.
- Owing to its low electrical conductivity, Bakelite is resistant to electric current.
Uses:
- They are used in manufacturing electrical switches and machine parts of electrical systems, due to its low electricity and high heat resistance
- It acts as a thermosetting polymer, as it has high strength, that retains its shape after extensive moulding.
- Phenolic resins are also extensively used as adhesives and binding agents. They are further used for protective purposes as well as in the coating industry.
- They are also used for making handles of utensils, it is one of the most common and important polymers that are used to make different parts of many objects.
Fibres
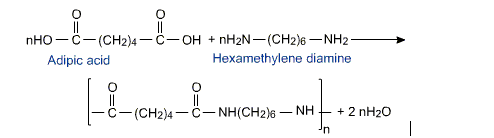
Nylon 6:6
Nylon is a synthetic polymer called as polyamide; this was first introduced by Wallace Carothers in 1935. It’s basically a polyamide that is obtained by polycondensation of adipic acid methylene diamine, and contains a total of 12 carbon atoms in each repeating unit.
The properties include:
- They are resistant to toughness and chemicals
- Good appearance and are thermally stable.
- They have high melting point of 268OCand are resistant to abrasion.
- Nylon 6,6 show high tensile strength and exhibits only half of shrinkage in steam.
- They are used in industrial products as they reduce moisture sensitivity in raw materials.
- Nylon 6,6 has a repeat unit with molecular weight of is 226.32 g/mol and crystalline density of 1.24 g/(cm)^3.
- They are amorphous solids and are soluble in boiling water and show large elastic properties.
- . Nylon 6,6 has long molecular chains resulting in more hydrogen bonds, creating chemical springs and making it very resilient.
- They are less susceptible to fading, once dyed they show high colourfastness
- They are not affected by water or alcohol.
Uses:
- The applications of Nylon 6,6 are: As Nylon is a light material, it is used in parachutes.
- They are water proof in nature, used extensively in swim wear
- They show high melting point, and make them resistant to heat and friction and used in places of wear and tear like airports, offices etc
- Nylon 6,6 being waterproof in nature is used to make machine parts. It is also used in the following like airbags, carpets, ropes. hoses etc. Hence Nylon 66 is a very useful creation by mankind.
Preparation of Nylon 6,6
The reactant mixture is heated under pressure, this kind of a process has
been developed to keep a check on the molecular mass of polymer that should be in a range of 12,000 to 20,000 amu.
|
Fig 5: formation of Nylon 6,6
The monomer caprolactam, that is obtained from cyclohexane (petrochemical)is used to prepare, these monomers are easily available. Due to its availability, it is used in polymerization that is carried out in the presence of water that hydrolyses caprolactam to an amino acid. Thus, the amino group of the amino acid reacts with caprolactam to form the polyamide polymer.
Filaments of Nylon-6 are obtained by melt-spinning of the polymer. The fibres are cooled by a stream of air.
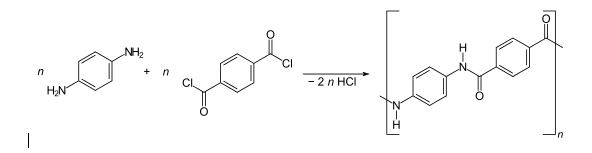
Kevlar
Kevlar is a synthetic fibre that are related to aramids, this was developed by Stephanie Kwolek in 1965. This fibre possesses a high strength and was used first commercially in 1970 in racing tires. Typically, it is spun into fabric sheets or ropes or fabric sheets that can be used as such or as an ingredient in composite material components.
Kevlar has many applications
- It is considered five times stronger than steel, due to its high tensile strength-to-weight ratio, they are extensively used in bicycle tires, racing sails and bulletproof vests
- It also is used to make modern marching drum heads to withstand high impact. It is used for mooring lines and other underwater applications.
Preparation
Kevlar is obtained by synthesizing a solution from monomers 1,4-phenylene-diamine (para phenylenediamine) and terephthaloyl chloride following condensation reaction that yields hydrochloric acid as a by-product. The result shows behaviour of liquid crystals and mechanical drawing orientation of polymer chains in the direction of fibres. The solvent used for polymerization is Hexamethylphosphoramide (HMPA), but for safety reasons, DuPont replaced it by a solution of N-methyl-pyrrolidone and calcium chloride.
|
Fig: 6 The reaction of 1,4-phenylene-diamine (para-phenylenediamine) with terephthaloyl chloride yielding Kevlar
Kevlar (poly paraphenylene terephthalamide) production is expensive due to the problems that comes up from using sulphuric acid. The water -insoluble polymer has to be kept in a solution during spinning and synthesis.]
Elastomers
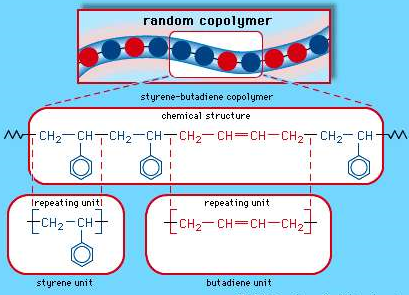
Buna S
Styrene-butadiene rubber (SBR), This is a copolymer that is produced from a combination of Styrene and butadiene, they are nothing but synthetic rubbers. And are known to be utilized more than any other synthetic rubber. They replace the natural rubber (polyisoprene) and are used in large scale in automobile and truck tires.
|
Fig: 7 The random copolymer arrangement of styrene-butadiene copolymer.
Each coloured ball in the molecular structure diagram represents a styrene or butadiene repeating unit as shown in the chemical structure formula.
SBR is known to have good abrasion resistance, they are resistant to cracks and do not undergo ageing soon., They degrade by atmospheric Oxygen and ozone and is weakened by hydrocarbons. SBR replace the natural rubber due to their economic utilities.
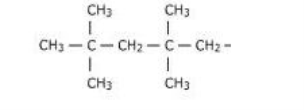
Butyl Rubber
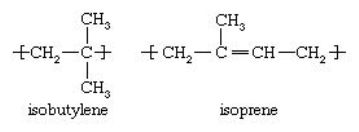
- This is a kind of rubber that is a copolymer of isobutylene and a small portion of isoprene
- They are also known as IIR. Where IIR stands for Isobutylene Isoprene Rubber.
|
Fig 8: Structure of a Butyl Rubber
Properties of butyl rubber:
- The rubber was commercially available first in 1943
- The properties of butyl rubber include good permeability and good flexibility that results in low levels of unsaturation between long polyisobutylene segments.
- The inner tubes of Tyre were the first major use of this rubber.
- In the 1950 and 1960 the development of halogenated butyl rubber was extended to a large extent as butyl rubber showed higher rates of curing and enabled vulcanization and also general rubbers like natural and styrene-butadiene.
- These important properties were used in the development of more tubeless tyres that retains air in the inner liner chemically bonded to the tire, the application of halo butyl for the inner liners has so far been the largest application. Both chlorinated (chlorobutyl) and brominated (bromobutyl) versions of halobutyl are commercially available.
- The butyl rubber is also good impermeable, ozone and weather resistant, vibration dampening, and stability make them good materials for pharmaceutical stoppers, construction sealants, hoses, and mechanical goods
USES:
- As fuel and lubricant additive
Polyisobutylene (in the form of polyisobutylene succinimide, PIBSI) shows good properties and is used as an additive in lubricating oils and motor fuels.
- As explosives
They often used by the explosives industry they form the binding agent in plastics explosives, they make the explosive insensitive to early detonation.
- In Sporting equipment
Butyl rubber is used for the bladders in sporting balls, e.g., Rugby balls, footballs, basketballs, netballs to provide a tough, airtight inner compartment.
- In Damp proofing and roof Repair
Butyl rubber sealant is used for damp proofing, rubber roof repair and for maintenance of roof membranes (especially around the edges).
It is used for repairing and waterproofing metal roofs.
- In Gas masks and chemical agent protection
Butyl rubber is one of the most robust elastomers when subjected to chemical warfare agents and decontamination materials. It is therefore used to create seals in gas masks and other protective clothing.
- In Medical Stoppers
Butyl and Bromo butyl rubber are commonly used for manufacturing rubber stoppers used for sealing medicine vials and bottles.
- In Chewing gum
Most modern chewing gum uses food-grade butyl rubber as the central gum base, which contributes not only the gum’s elasticity but an obstinate, sticky quality which has led some municipalities to propose taxation to cover costs of its removal.
- In Tyres
Butyl rubber and halogenated rubber are used for the inner liner that holds the air in the tyre
Preparation
Butyl rubber, is a synthetic rubber, and also a copolymer of isobutylene and isoprene hence it is also called as isobutylene-isoprene rubber. The butyl rubber was first produced by William sparks and Robert Thomas in 1937.
Isobutylene is obtained from refinery streams and in presence of sulphuric acid, they are formed. Isobutylene(C[CH3]2=CH2) is a branched alkene that has four carbon, they are tasteless and odourless Isobutylene and isoprene (CH2=C[CH3]-CH=CH2) are obtained by the thermal cracking of natural gas or of the lighter fraction of crude oil.
Infect at normal temperature isoprene is a volatile liquid and Isobutylene is a gas. Butyl rubber is produced copolymerization of 98% of isobutylene in a solution with a low concentration of 2% isoprene. The compound Isobutylene is refrigerated at low temperatures and then diluted with methyl chloride, the. Reaction is started on adding low concentration of isoprene in the presence of aluminium chloride resulting in the copolymerization of compounds where single unit molecules join together to form huge multiple unit molecules
|
Butyl rubber which contains small amount of isoprene and because polyisobutylene’s pendant group are arranged in a regular order along the polymer chains and because the chains crystallize rapidly on stretching, it is strong as natural rubber.
Silicone rubbers
Silicone rubber are elastomers it’s a polymer consisting of silicon, carbon and hydrogen and oxygen. Silicone rubbers are widely used in industry, they are often in one-or two-part polymers and to reduce cost they may contain fillers.
Properties
Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C (−67 to 572 °F) while still maintaining its useful properties.
Silicone is flexible and used in manufacturing and shaping in a variety of products. including: voltage line insulators, automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in-home repair and hardware with products such as silicone sealants.
Preparation
To prepare silicone the atoms must be first isolated from silicon dioxide compound silica. This is achieved by heating huge volumes of quartz sand to extremely high temperatures, often up to 1800oC, silica then undergoes a series of reactions and is combined with methyl chloride and heated. It is then distilled into a polymerised siloxane known as polydimethylsiloxane. This compound can then be polymerized. This is done using a variety of techniques depending on the use of the final product. The raw silicone compound is then often combined with pigments, any additives required and combine with the catalyst for injection moulding or extrusion. Curing is the final stage in the production process.
Uses
Silicone rubber is used in a variety of departments, they are useful in automotive. And also, in baking, cooking and food storage products, they are used in the garment industry for sportswear footwear etc.
Liquid silicone rubber is also manufactured for application in sciences like (syringe pistons, closure for dispensing system, gaskets for IV flow regulator, respiratory masks, implantable chambers for IV administration), cosmetic products (Mascara brush, make-up packaging, make-up applicator and lipstick moulds) and optics products.
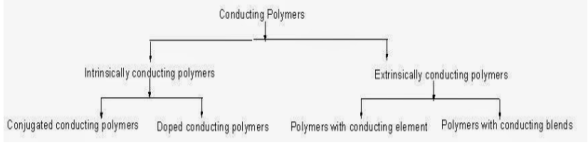
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are polymers that are organic in nature and also conduct electricity., such polymers can be semiconductors or may show metallic conductivity. The conductive polymers show one of the biggest properties of dispersion, they are not thermoplastic in nature and have the advantage of being process able. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. Methods like organic synthesis and dispersion techniques can be implemented to enhance the electrical properties in conductive polymers.
Classification of Conducting polymers
|
Mechanism of conduction in polyacetylene
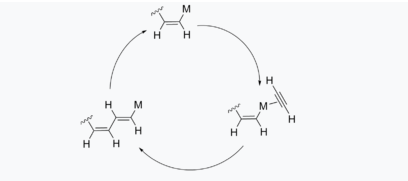
One of the most common methods uses a Ziegel-Natta catalyst Ti (OiPr)4/Al(C2H5)3 along with gaseous acetylene. By varying temperature and catalyst loads the structure and properties of the final polymer can be controlled. Mechanistic studies suggest that this polymerization involves metal insertion into the triple bond of the monomer.
|
Fig 9 Insertion mechanism for polyacetylene
Shirakawa and his co-workers were able to synthesize polyacetylene into thin films than insoluble black powder by varying the catalyst loading and the apparatus. Such thin films were obtained by coating the walls of the reaction flask and with a solution of Ziegler–Natta catalyst and adding gaseous acetylene, under inert conditions. Later Enkelmann and co-workers improved further the synthesis of polyacetylene by changing the catalyst to a Co (NO3)2/NaBH4 which was stable to both oxygen and water.
Applications of conducting polymers
- They are used in polymer rechargeable batteries, with repeated oxidation and reduction.
- They are used in polymer cell batteries, as they are flexible and low of cost.
- They are also used as active layers of gas sensors e.g., derivative of polyaniline.
- Chemical receptors widely use conducting polymers, and is used as a substrate insulate
- Used as corrosion inhibitors.
Some examples are given as follows: -
Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV): It is derived by combining 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid, in which monomers are cross-linked by an ester linkage. It decomposes to form carbon dioxide and water. The compound is brittle and is utilised in drug production and bottle manufacture.
A polymer that can be decomposed by bacteria is called a biodegradable polymer.
The biodegradable polymer are the polymers which are degraded by the micro-organism within a suitable period so that biodegradable polymers & their degraded products do not cause any serious effects on the environment. They degrade by enzymatic hydrolysis & oxidation. The decomposition reactions involve hydrolysis (either enzymatically induced or by non –enzymatic mechanism) to non- toxic small molecules which can be metabolized by or excreted from the body.
The common examples of aliphatic biodegradable polymers are polyglycolic acid(PGA), Polyhydroxy butyrate (PHB), Polyhydroxy butyrate’s-co-beta hydroxyl valerate( PHBV), Polycaprolactone(pcl), Nylon-2-nylon-6.
These polymers are used mainly for medical goods such as surgical sutures, tissues in growth materials, for controlled drug release, plasma substitutes etc. They are also used in agriculture materials, such as films, seed coatings, fast food wrappers, personal hygiene products etc.
Preparation, properties and Applications of polylactic acid
Polylactic acid, or polylactide (PLA) is a thermoplastic polyester with backbone formula (C3H4O2)n or [–C(CH3)HC(=O)O–]n, formally obtained by lactic acid condensation C(CH3)(OH)HCOOH with loss of water (hence its name). Polylactic acid can also be obtained by ring-opening polymerization of lactide [–C(CH3)HC(=O)O–]2, the cyclic dimer of the basic repeating unit.
Preparation
This monomer is specifically made from starch of fermented plants that include sugarcane, corn, cassava or beet pulp
Several industrial can afford this PLA due to its high molecular weight. LA is obtained by two monomers which are lactic acid and cyclic di-ester, polymerization of ring opening of lactide with various catalysts like tin octoate in a suspension or a solution. The metal-catalysed reaction tends to cause racemization of the PLA, reducing its stereoregularity compared to the starting material (usually corn starch).
Properties
Chemical properties
Distinct forms of PLA are present due to its chirality. poly-L-lactide (PLLA) is the product resulting from polymerization of L, L-lactide (also known as L-lactide). PLA is soluble in solvents, hot dioxane, tetrahydrofuran and benzene.
Physical and mechanical properties
- PLA polymers have a melting point of 130-180 °C and are amorphous glassy polymers to semi-crystalline and highly crystalline polymer that have a glass transition of 60-65oC and a tensile modulus 2.7–16 GA.
- Heat-resistant PLA can withstand temperatures of 110 °C. The basic mechanical properties of PLA are between those of polystyrene and PET.
- The melting temperature of PLLA can be increased by 40–50 °C and its heat deflection temperature can be increased from approximately 60 °C to up to 190 °C by physically blending the polymer with PDLA (poly-D-lactide).
Applications
- PLA is used as a feedstock material in desktop printers
- PLA-printed solids are put into plaster moulds and burnt in a furnace the resulting gap can be filled with molten metal. This is known as "lost PLA casting".
- PLA can degrade into innocuous lactic acid, so it is used as medical implants in the form of anchors, screws, plates, pins, rods, and as a mesh Depending on the exact type used,
- The strength characteristics of PLA and PLLA implants are well documented. PLA can also be used as a decomposable packaging material, either cast, injection-moulded, or spun. Cups and bags have been made from this material. In the form of a film, it shrinks upon heating, allowing it to be used in shrink tunnels
Key takeaway
A small molecule that can be combined with other small molecules to make polymers, Polymers are giant molecules formed from addition or condensation reactions and can be classified as either biological or synthetic polymers.
Synthetic polymers are human-made polymers. They can be classified into four main categories: thermoplastics, thermosets, elastomers, and synthetic fibres. They are commonly found in a variety of consumer products. Various main chains and side chains are used to make different synthetic organic polymers.
References:
1.Introduction to Polymers by Robert Young and Peter Lovell
2. Polymer Chemistry by Paul Hiemenz and Timothy Lodge.
3.Polymer Science by N.V Viswanath, Jayadev Sridhar , V.R Gowarikar
4.Polymer Science and Technology by Joel Fried.