Water is a chemical compound consisting of two hydrogen atoms and one Oxygen atom. The name water typically refers to the liquid state of the compound. The solid phase is called as ice and the gas phase is called as steam. Under specific conditions, water also forms a supercritical fluid. Water is the main compound found in living organisms. Approximately 62 percent of the human: body contains water. The word "water" comes from the Old English word water or from the Proto-Germanic watar or German Wasser. All of which mean "water" or "wet." The boiling point of water is 99.98 degrees C (211.96 degrees F; 373.13 K).
Water is amphoteric. Which means, it can act as both an acid and as a base.
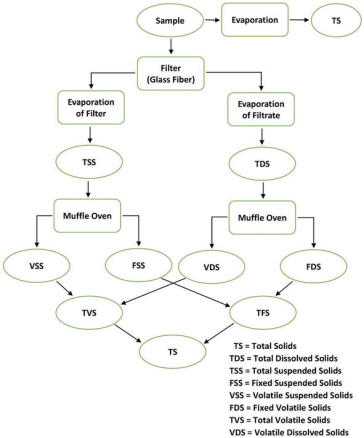
Solids exist in water in either forms, a solution or in suspension. The two types of solids can be identified by using a glass fiber filter through which water sample passes through. By definition, the suspended solids are retained on the top of the filter and the dissolved solids pass through the filter with the water.
When the filtered portion of the water sample is placed in a small dish and then evaporated, the solids form as a residue. This material is called total dissolved solids or TDS.
Total solid (TS) = Total dissolved solid (TDS) + Total suspended solid (TSS)
Water can be classified by the amount of TDS per liter as follows:
- freshwater: <1500 mg/L TDS;
- brackish water: 1500–5000 mg/L TDS;
- saline water: >5000 mg/L TDS.
The residue of TSS and TDS after heating to dryness for a specified period of time and at a specific temperature is defined as fixed solids. Volatile solids are those solids lost on ignition (heating to 550°C).
These necessary measures help the operators of the wastewater treatment plant because they roughly approximate the amount of organic matter that exists in the total solids of Industrial waste, activated sludge, and waste water. Fig. 3 describes the interrelationship of solids found in water. They are calculated as follows:
- Total solids:
|
Figure 1: Interrelationship of solids found in water.
Total solids(mg/L) = [(TSA – TSB)] × 1000/sample(mL)
4.10 Calculation of hardness of water in all units
One of the prime factors that establishes the quality of a water supply is its degree of hardness. Hardness is defined as amount of calcium and magnesium ion content present in water. Since most experiments do not distinguish between Ca2+ and Mg2+, and most hardness is caused by carbonate mineral deposits, hardness is usually derived as parts per million (ppm) of calcium carbonate (by weight). if water is supplied with a hardness of 100 ppm that consists an equivalent of 100 g of CaCO3 in 1 million g of water or 0.1 g in 1 L of water.
Estimation of Hardness of water by EDTA Method and Alkalinity Method
Temporary Hardness of Water:
When calcium carbonate and magnesium is present in water, the water is temporarily hard. This hardness is usually removed y boiling
When water is boiled the soluble salts of Mg (HCO3)2 is converted to Mg (OH)2 this is insoluble in water and therefore gets precipitated and is removed later by filtration. Thus, soft water is obtained.
The four acid oxygen sites and the two nitrogen atoms have unshared pair of electrons, which can form bonds with a metal ion forming a complex ion or coordination compound. The complex that is formed is quite stable, and the conditions of its formation can easily be controlled so that it is ready for selection for a particular metal ion. when a titration to determine the concentration of a metal ion is carried out, the added EDTA quantitatively combines with the cation to form the complex. The endpoint is reached when essentially all of the cation has reacted. In this analysis a solution of EDTA will be standardize by titration against a standard solution made from calcium carbonate, CaCO3. The EDTA solution can then be used to determine the hardness of an unknown water sample. Since both EDTA and Ca2+ are colourless, therefore a special indicator is used to detect the end point of the titration. The indicator mostly used is called Eriochrome Black T, which forms a very stable wine-red complex, MgIn–, with the magnesium ion. A small amount of this complex will be present in the solution during the titration. As EDTA is added, the complex free Ca2+ and Mg2+ ions, leaving the MgIn– complex alone until essentially all of the calcium and magnesium are converted to chelates. At this point EDTA concentration will increase marginally to displace Mg2+ from the complex indicator; the indicator reverts to its uncombined form, which is sky blue, thus establishing the end point of the titration. The titration is carried out at a pH of 10, in a NH3/NH4 + buffer, the equations for the reactions which occur during the titration are:


 Titration reaction: HY3–(aq) + Ca2+(aq) CaY2–(aq) + H+ (aq) (also for Mg2+) End point reaction: HY3–(aq) + MgIn– (aq) MgY2–(aq) + HIn2–(aq)
Titration reaction: HY3–(aq) + Ca2+(aq) CaY2–(aq) + H+ (aq) (also for Mg2+) End point reaction: HY3–(aq) + MgIn– (aq) MgY2–(aq) + HIn2–(aq)
As the indicator requires a trace of Mg2+ to operate properly, a little magnesium ion will be added to each solution. The effect of the added Mg2+ can be subtracted by titrating a blank.
A simple way to calculate the hardness in water.
A sample of hard water contains 1mg cacl2 and 1 mg mgcl2 per
litre . calculate the hardness of water in terms of caco3 present in per 106 parts of water. Hardness of water in terms of Caco3= (molar mass of hardness causing substance*molecular weight of Caco3)/ (molecular weight of hardness causing substance. Thus, cacl2 hardness = 1mg*100/111 = 0.900ppm for Mgcl2 Hardness = 1mg*100/95=1.052ppm Total hardness of water in terms of Caco3 = 0.90+1.052= 1.952ppm
|
Alkalinity
Waters that have moderate to high levels (50 mg/L or greater) of total alkalinity and total hardness usually have a neutral to slightly basic pH. At this stage the pH is more stable and does not change greatly throughout the day because the presence of carbonates and bicarbonates neutralize, or "buffer," the carbon dioxide and other acids in the water.
Alkalinity is a measure of the capacity of water to neutralize hydrogen ions or acids. Alkalinity is otherwise to as "Carbonate hardness". Alkalinity acts as a buffer when any changes are made to the water's pH value. The Alkalinity in the water will help keep the pH of water stabilized. The drinking water and all water should be a pH of 7 which neutral.
Procedure of Alkalinity Test
The titration tube up is filled up to the 5ml line with water.
- Then one Bromocresol Green-Methyl Red tablet is added into water. Cap and shake until the tablet disappear. The water might turn blue-green. If the water turns pink, the water doesn't have alkalinity.
- Fill the direct-reading titration with the Alkalinity Titration Reagent B. Then fill the titration when the plunger is up to the zero-graduation mark. After the plunger is added up to the zero-graduation mark, put the titration in the centre hole of the titration tube cap.
- Titrate the plunger slowly till the titrate sample which is blue-green colour changes to pink. The amount of Reagent B that is consumed into the titration until it turns pink is the amount of alkalinity in the water. It is measured my parts per million (ppm) out of 200 ppm.
Ion exchange method including Zeolite method
Ion exchange process to soften water, using cations or anions. This is done by exchanging cations or anions with the calcium and magnesium ions in hard water. This process involves a reversible chemical reaction. However, we can use this technique only in dilute solutions. The equipment that we use for this purpose is ion exchangers.
Types
There are two types;
- Cation exchangers – use zeolite, greensand, sulfonated coal, etc. as the exchanging material.
- Anion exchangers – uses metallic oxides, synthetic resins, etc.
The materials used in cation exchangers include either weak acids or strong acids. Strong acid cation exchangers mainly contain sulfate functional groups. Weak acid cation exchangers mainly contain carboxyl groups. The materials that are used in anion exchangers include either weak bases or strong bases. Moreover, there are several categories of ion exchange process used for water softening, Dealkalization and demineralization. The ions that are part of the exchange process (the ions that exchange with the calcium and magnesium cations in hard water) include sodium ions, hydrogen cations, chloride anions and hydroxyl anions.
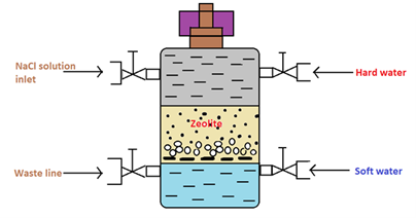
- Zeolite process
There are two types of zeolite used in this process they include natural and synthetic zeolite. The natural form is found to be porous and synthetic form is a non-porous zeolite. however synthetic form possesses a high exchange capacity per unit weight than the natural form.
|
Fig 2:Cylinder Containing the Zeolite Bed
Process
In the water softening process, hard water is passed through a bed of zeolite (inside a cylinder) at a specified rate. Then the cations that cause the water-hardening will remain on the zeolite bed because these cations exchange with the sodium cations of zeolite. Therefore, the water coming out of this cylinder contains sodium cations rather than calcium and magnesium cations.
After some time, the zeolite bed gets exhausted, the water flow is stopped and treat the bed is treated with concentrated brine solution (10%) in order to regenerate the zeolite. The bed is treated with a brine solution, as it washes away all the calcium and magnesium ions, by exchanging them with sodium ions in a brine solution. hence, this treatment regenerates the zeolite.
Reverse Osmosis
Distillation and reverse osmosis are the most widely used two non-chemical methods of water softening.
To remove the temporary hardness from 5 L5 L of H2OHX2O 5.6 mg5.6 mg of lime is required. Calculate the degree of hardness due to Ca (HCO3)2Ca (HCOX3) X2 present in water
Ca (HCO3)2+CaO⟶2CaCO3+H2O
Since 5.6 mg5.6 mg of lime is required, amount of calcium bicarbonate present is 16.2×10−316.2×10−3.
Molarity = 16.2×10−3/ (162×5)
=0.002 M=0.002 M.
This is equivalent to the concentration of calcium carbonate.
ppm of calcium carbonate = 0.002×10−2×100×106×10−3=2000.002×10−2×100×106×10−3=200
Therefore, degree of hardness is 200 ppm
2.3 Specifications of Potable water
Bureau of Indian Standards (BIS) provides standard specifications for potable water (water that is safe to drink) (BIS-10500-1991, drawn up in 1983 and revised periodically.
The various parameters covered include odour, dissolved salts, pH, hardness, alkalinity, elemental compounds such as iron, manganese, sulphate, nitrate, chloride, fluoride, arsenic, chromium, copper, cyanide, lead, mercury, zinc and coliform bacteria.
The standard categorises various characteristics as essential or desirable. It mentions the desirable limit and indicates its background so that the implementing authorities may exercise their discretion, keeping in view the health of the people, adequacy of treatment etc.
2.4 Chlorination sterilization
Chlorination is a kind of sterilization of water, it is one of the most and cheapest way to sterilize water. Chlorine is added to water in the form of chlorine gas, sodium hypochlorite or calcium dioxide. When chlorine is injected, they form several chemicals like hypocholorous acid.
Chlorine sterilization
To treat municipal and wastewater treatment Chlorine is one of the mostly used disinfectant, Chlorine destroys a number of pathogens and controls most of the organisms. Chlorine can also manganese ammonia iron and nitrogen from water. Chlorine is a toxic gas and acts as a quick oxidizing agent. Chlorine is easily affected by pH, and needs to be controlled. The reaction that occurs is Chlorine reacts with water and form hypochlorous acid that is further break into nascent oxygen. Both of them are powerful germicide.
Cl2 + H2O → HOCl + HCl ; HOCl → HCl + [O]
Breakpoint chlorination is defined as the point where sufficient chlorine has been added to a quantity of water to enable disinfection. In other words, it is at this point that all the undesirable contaminants are removed from the water. It is at this breakpoint that, all the chlorine that is added to the solution gets consumed by the chemical reactions of the contaminants, that results in water having no free availed chlorine (FAC).
In waste water treatment, breakpoint chlorination involves removing of ammonia from a solution, that changes to an oxidised volatile form. Increased amounts of chlorine residue are produced by adding chlorine to water that contains ammonia or organic matter that contains nitrogen.
This process involves the continuous addition of chlorine to water, till a point where chlorine brings aout a suppression in the rate of corrosion successfully., that makes the diffusion process difficult due to rust.
Excessive amount of chlorine causes detrimentation to the human tissues. In a reaction Chlorine can undergo strong oxidation causing hydrogen to separate from water, this hydrogen combines with chlorine to form hydrogen chloride and nascent oxygen, that causes damage in tissues.
Generally, in swimming pools, breakpoint chlorination has a certain amount of free chlorine that is used to fully remove combined chlorine from the pool water. It is necessary to avoid the unsuccessful termination
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the corrosion.
All metals can corrode. Some metals, like pure iron, deteriorate very fast. Stainless steel, and, metals that combines with iron and other alloys, is slower to corrode and is therefore used more efficiently.
All small group of metals, are called the Noble Metals, and show much less reaction than others. As a result, they deteriorate rarely. They are, in fact, the only metals that are found in nature in their purest form. The Noble Metals, not unexpectedly, are often very valuable. They include gold, rhodium, silver, palladium, and platinum.
- Dry or Chemical theory of corrosion: The direct reaction of Atmospheric gases (like halogens, oxygen, oxides of nitrogen and sulphur, fumes of chemicals and with metals and hydrogen sulphide), on the surface of the metal causes corrosion. Oxygen is the most responsible for corrosion of metals than any other gases or chemicals. This process occurs in the absence of moisture and affects the metal surface directly. Corrosion products are formed at the site of corrosion.
The three types of corrosion are
Oxidation Corrosion, Liquid metal corrosion and Corrosion by other gases.
2. Wet or Electrochemical theory of Corrosion: This corrosion occurs in metals that come in direct contact with a conducting liquid of two different metals are dissolved in a solution partly. There is formation of galvanic cell on the metal, as part of the metal acts as an anode and the rest is the cathode, the chemical in the solution along with the humidity acts as the electrolyte. Oxidation takes place in such conditions resulting in corrosion at the anode surface and reduction occurs at the cathode surface of the metal. In this case corrosion occurs at the anode but rust gets deposited on the cathode.
Electrochemical Corrosion and Mechanism
Corrosion is an electrochemical reaction that appears in many forms, that includes chemical corrosion and atmospheric corrosion, the most common one being the latter one. Rust is formed when acidic substances including water come in contact with metals like iron and steel. Once the iron and steel particles are exposed to oxygen and moisture (e.g., humidity, vapor, immersion) resulting in the formation of rust. When steel is exposed to water, the iron particles get lost in the water’s acidic electrolytes. The particles then become oxidized, resulting in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and these electrons flow through the steel to another area of the steel known as the cathodic area.
Oxygen causes these electrons to rise up and form hydroxyl ions (OH). The hydroxyl ions react with the FE⁺⁺ to form hydrous iron oxide (FeOH), better known as rust. Therefore, the Iron particles become a corrosion pit. And correspondingly the product is called corrosion product namely Rust.
Corrosion can happen at any rate, depending on the environment that the metal is in. However, since atmospheric corrosion is so widespread, it is recommended to take effective precautionary measures when it comes to corrosion prevention.
Waterline Corrosion
This mainly occurs when there is a difference in the oxygen concentration between the water and the atmosphere. The material that is more exposed to air forms the cathode and the portion that is less exposed to oxygen (the portion dipped in water) forms the anode. The presence of anode and cathode allows an oxidation reaction to occur. This ultimately causes the area of the substrate submersed in water to become oxidized and corrode
Pitting Corrosion
Pitting corrosion is another example of corrosion where the local area of the metal can be attacked leading to the formation of holes in the metal. This type of corrosion can lead to stress and also cracking of the material, an example for which is the collapse of the Silver Bridge in West Virginia, the USA in 1967.
Pitting corrosion occurs when a small area of the metal gets affected by the environment and forms the anode and the rest of the metal forms the cathode, leading to a galvanic corrosion, that begins at the surface of the metal and gradually spreads downwards resulting in the structural failure of the metal.
Damages Caused by Pitting Corrosion
Pitting corrosion can lead to the formation of pits on the metal. The shape of these pits can vary from shallow and wide to deep and narrow. An illustration depicting the different shapes that a cavity formed on the metal due to pitting corrosion can take.
Main factors which affect corrosion are
1. The more the metal shows its reactivity, the possibility of the metal getting corrode increases.
2.The corrosion occurs faster when the impurities help in setting up voltaic cell.
3. The rate of corrosion is also affected by the presence of electrolytes in water.
4. The rusting of Iron is increased if the amount of carbon dioxide is present in more amounts in water.
5.The rate of corrosion can however be reduced when the surface of Iron is coated with layers of metal that are actually more active than the Iron itself.
6. An increase in temperature (within a reasonable limit) also increases the rate of corrosion.
Cathodic protection methods
Cathodic Protection
Cathodic protection in this process the main principle is to switch from the works by changing over undesirable anodic (active) sites on to a metal's surface to cathodic (passive) destinations in the presence of a restricting current. This restricting current provides free electrons and also supplies power to the nearby anodes,
Cathodic protection can, the presence of galvanic anodes. This process is also known as conciliatory form, this technique utilises metal anodes together with the electrolytic condition, to make themselves strong (erode) so as to secure the cathode. This technique proves to save the metals galvanic corrosion.
Though the metal that requires protection can differ, the conciliatory anodes are largely made up of magnesium, or zinc, metals that have the most negative electro-potential. The arrangement of galvanic setup gives a glimpse of the distinctive electro-potential - or integrity - of metals and amalgams.
In a conciliatory framework, the anode should be supplanted regularly as the metallic particles move from the anode to the cathode, which drives the anode to erode more rapidly. The second technique for cathodic security is alluded to as awed current protection.
This strategy, which is regularly used to ensure covered pipelines and ship bodies, requires an elective wellspring of direct electrical current to be provided to the electrolyte.
Sacrificial Anode
A sacrificial anode is also known as a galvanic anode
The mechanism that occurs in this process is very similar to that of an electrochemical system. In this technique the metal that is protected is placed on the cathode side and then a more reactive metal or alloy (having a larger potential difference than the protected metal) is chosen and connected to the protected metal as an anode. The redox reaction will proceed spontaneously. As the reaction proceeds the sacrificial metal gets consumed as oxidation reaction occurs at the anode, simultaneously reduction reaction occurs on the cathode, thus preventing the metal from erosion. Thus, corrosion on the protected metal is successfully shifted to the anode, protecting the metal.
Sacrificial anodes are normally supplied with either lead wires or cast-m straps to facilitate their connection to the structure being protected. The lead wires may be attached to the structure by welding or mechanical conn
The materials used for sacrificial anodes are either relatively pure active metals, such as zinc or magnesium, or are magnesium or aluminium alloys that have been specifically developed for use as sacrificial anodes.
Advantages of using sacrificial anodes:
- Can be used where there is no power
- Lower initial cost
- Less supervision required
- Comparatively simple installation and additional anodes can easily be added if the initial installation proves to be inadequate
Sacrificial anodes are used to protect:
- Hulls of ships
- Water heaters
- Pipelines
- Distribution systems
- Above-ground tanks
- Underground tanks
- Refineries
The anodes in sacrificial anode cathodic protection systems must be periodically inspected and replaced when consumed.
Impressed current methods
In this technique, protection from corrosion is obtained by electrochemical means. It is a type of cathodic protection, where the dissolution of anodic structures is reduced, that occurs by decreasing the difference the potential energy, and also when the metal surface is placed in a conductive electrolyte such as water and the cathodic and anodic metal surface sites face less reduction.
Theoretically, on observation the impressed current cathodic protection is obtained during the stage where open circuit potential of cathodic areas gets polarized into the same circuit potential of anodic sites. The key in impressed current protection is to turn the whole structure cathodic in nature, or make it a current receiver rather than a current provider.
An impressed current protection system can offer protection to structures and metals like:
- Fuel pipes
- Steel
- Water
- Hulls
- Tanks
- Water heaters
- Oil casings
It can also serve as a metal reinforcement in the case of concrete buildings, structures and bars. The method can be applied in galvanised steel, where the steel parts are protected from rusting by sacrificial zinc coatings.
Utilizing this type of protection offers the following practical benefits:
- Infrastructure cost for corrosion is reduced.
- Infrastructure failure incidents become rare.
- Product loss from corrosion is reduced.
- Lower downtime in terms of emergency repairs
- Increased service life of infrastructures
- Enhanced quality of infrastructure service
- Increased security and health to underlying infrastructures
An impressed current system serves as an ideal protection system for bigger structures that are not capable of delivering ample current to offer protection. This system is mainly composed of anodes attached to a power source (DC), which can be a rectifier connected to power (AC). In the absence of an AC power supply, other power sources may be used like wind power, solar panels and gas power. The anodes in the impressed current protection system can be found in various sizes and shapes. The most common ones are tubes and solid rods, while there are also continuous ribbons made from various materials like:
Surface Coating
This is the most economical method and simple wherein the membrane surface is coated by a layer. This method can be carried out on a large scale in industries.
The most common coating materials include tin, lead, aluminium etc. The principle in this method includes formation of a transition layer from the various alloy compositions. To the substrate inter-metallic compounds of the two metals are normally present and on the exterior are alloys that consist of solid solution alloys that predominantly form the coating materials.
Surface coating is a process where a substance is applied to other materials bringing about changes like gloss, colour, resistance to chemical attacks or permeability, however the bulk properties of the material remains unchanged. Production of surface coating by any method depends primarily on two factors: the cohesion between the film forming substances and the adhesion between the film and the substrate. The development of science and technology revolutionized the surface coating industry
- Hot-dip galvanizing is a form of galvanization where the iron or steel is immersed in a bath that consists of molten zinc at a temperature around 449o C (840 °F), this helps in producing a multi-layer coating of zinc-iron alloy and zinc metal. The layer is also corrosion resistant, when steel is immersed in zinc, a reaction which is metallurgical occurs between the iron and the molten zinc.
- Galvanizing, it is a process that involves the protection of steel and iron from exposure to the atmosphere leading to rusting, this is however prevented by the application of zinc coating. Proper coating by galvanization can protect the material from the atmosphere and corrosion for nearly 15 to 30 years, however is pores develop in the coating galvanic or electrolytic action occurs.
Key takeaway
Water chemistry analysis is often the groundwork of studies of water quality, its alkalinity, purification methods, softening methods and its determination of hardness through various methods, pollution, hydrology and geothermal waters. Analytical methods routinely used can detect and measure all the natural elements and their inorganic compounds and a very wide range of organic chemical species using methods such as gas chromatography and mass spectrometry, making water available for all life activities in its purest from.
Corrosion is a process of formation of the compound of pure metal by the chemical reaction between metallic surface and its environment
References:
1. Industrial Chemistry, B.K.Sharma,Goyal
2. Chemistry for Engineers, Rajesh Agnihotri, Wiley
3. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
4. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
5. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand
6. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
7. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand