Unit - 5
Enzymes
All biological reactions that occur within human cells and other organisms are dependent on enzymes. Their ability to act as catalysts and enable biological reactions to occur usually in milliseconds or even faster. In 1995, Wolfenden reported that without a particular enzyme, the enzymes are very important in a biological transformation he deemed "absolutely essential" in creating the building blocks of DNA and RNA
The enzyme is essential for both animal and plant life on the planet," Wolfenden said. According to him " The Evolution has to analyse or surpass the fact that enzymes that are involved in analysis have overcome a huge barrier of all kinds and the reaction that occurs has a life of 2.3 billion years”
Biologists appreciate that the enzyme evolution as a productive catalyst by understanding as to how long reactions would take place without enzymes, Wolfenden said. It also helps scientists to compare the naturally occurring and enzymes with artificial catalysts produced in the laboratory.
"Without catalystslife would be impossible and the reactions would take much longer to happen, beginning from microbes to humans respectively," he said.
A substance that has the ability to increase the rate of a chemical reaction without it being changed or altered by the reacting chemicals or being consumed by the is called a catalyst. The action performed by a catalyst is called catalysis. The reactions or processes would be relatively slow, Catalysts are therefore used by chemists to speed up chemical reactions which would otherwise take a long time to complete, however the exact mechanisms of catalytic actions are not completely known yet, but in general the catalyst combines with the reacting chemicals to form a temporary new substance or forms a complex with the substance and then emerges in its original form at the end or completion of the process.
Catalysts can occur in one or more forms they may be gases, liquids, or solids, and their catalytic activity is classified as either homogeneous or heterogeneous. A homogeneous catalyst is one where the molecules are dispersed in the same phase (usually gaseous or liquid) as the reacting chemicals. On the other hand, a heterogeneous catalyst is one whose molecules are not dispersed in the same phase as the reacting chemicals. In Heterogeneous catalysts they occur mostly solid form, and the reacting chemicals are usually liquids or gases that are adsorbed onto the surface of the catalyst. Good solid catalysts arehaving the property of being usually highly porous, with surface areas of several hundred square meters per gram. Palladium and finely divided platinum in an automobile’s catalytic converter, for example, efficiently convert exhaust pollutants into nitrogen, carbon dioxide, and water.
Enzymes are alsocalled biological catalysts. They are present as proteins in plants and animals that catalyse the biochemical reactions required for life. For example, the enzymes in saliva, accelerate the conversion of starch to glucose, doing in minutes what would otherwise take weeks without the presence of the enzyme.
Monitoring the rate of an enzyme- that catalyses a reaction is called ‘enzyme kinetics’. The kinetics of an enzyme-catalysed reaction can indirectly provide information about the mechanism of catalysis. The rate or velocityof a reaction is the change in the concentration of reactant or product per unit of time
- The rate of enzyme reaction is measured by the amount of substrate changed or amount of product formed during a period of time.
- The rate is determined by measuring the slope of the tangent to the curve in the initial stage of the reaction. The steeper the slope, the greater is the rate.
- If enzyme activity is measured over a period of time, the rate of reaction usually falls, most commonly as a result of a fall in the substrate concentration.
- The rate of reaction is proportional to the enzyme concentration provided that the substrate concentration at high level.
- If the enzyme concentration is increases, the rate of reaction increases.
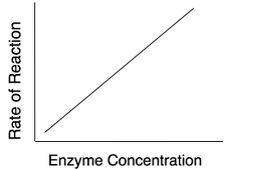
Fig1: For a give enzyme concentration, the rate of reaction increases with increase in substrate concentration until all the available active sites are occupied by the substrates.
Once all the active sites are used up, the rate of reaction remains constant with increase in substrate concentration. Therefore, the theoretical maximum rate is never quite obtained. The extra substrate has to wait until the next enzyme/substrate complex release product before it takes part in another reaction.
- Under constant other factor, pH affects the rate of reactions.
- Is Optimum pH being the pH at which the rate of enzyme-controlled reaction is maximum pH which is different for different enzymes.
- Rate of reaction decreases when the pH is either increased or decreased from its optimum value. The ionic charge of acidic or basic groupsare altered with change in pH and therefore disrupt the ionic bonding that helps to maintain specific shape of enzyme
- Thus, change in pH leads to alteration of enzyme shape including the active site.
- If extreme pH is introduced then it will denature the enzymes.
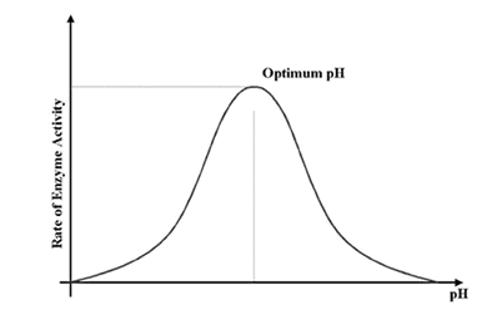
Fig 2: The rate of enzyme activity is maximum, at optimum pH.
An enzyme molecule binds to the substrate leading to changes in its conformation to reduce the activation energy of the substrate molecules. This leads to a faster rate of reaction when compared to the reaction in the absence of the enzyme in the reaction.
A specific enzyme is capable to catalyse a reaction by lowering the activation energy required to complete the conversion of a substrate into its end product.
The energy required to initiate particular reaction is called the activation energy on the other hand simply put, is the energy required to initiate a particular reaction, different enzymes do it in a few different ways.
But, the most common way that occurs in a reaction is when an enzyme binds to its substrate, certain covalent and ionic chemical compounds get molecularly bound at thislevel. When this binding occurs, it changes the conformation of these particular sites in such a way that molecules show up in different shapes.
In chemistry the changein shape of a compound temporarily can induce changes in certain properties such as aligning polarity of these molecules and making them slightly acidic or basic nature.
In general, enzymes are proteins produced by living cells, they act as catalysts in severalbiochemical reactions. A catalyst also affects the rate of a chemical reaction. In the presence of an Enzyme the cell can carry out complex chemical reactions at relatively low temperatures through enzyme activity. In an enzyme-catalysed reaction, the substance to be acted upon (the substrate = S) binds reversibly to the active site of the enzyme (E). The result thatforms such a temporary union shows a reduction in the energy required to activate or proceed the reaction of the substrate molecule so that the end products (P) of the reaction are formed.
In summary: E + S —> ES –> E + P
In this case the enzyme is not changed in the reaction and can even be recycled to break down into additional substrate molecules. For every reaction that takes place each enzyme is specific because its amino acid sequence is unique and causes it to have a specific three-dimensional structure. The active site is the portion or the site of the enzyme that interacts with the substrate, the activity of the enzyme is affectedwhen any substance that blocks or changes the shape of the active site.
Enzymes are usually protein molecules that are capable of manipulating other molecules— namely the enzymes' substrates. In an Enzymatic reaction a series of steps occur where the target molecules bind to an enzyme's active sites and are transformed into the final products.
E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P
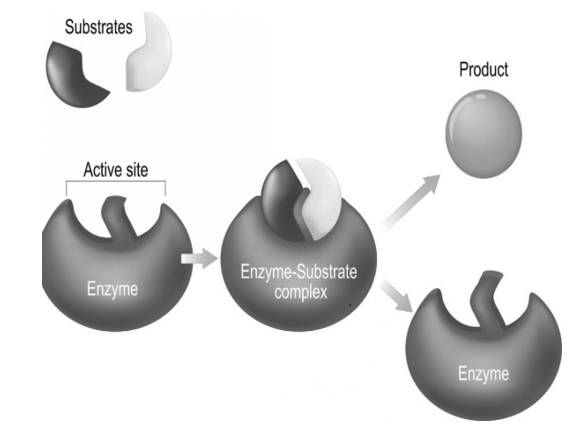
Fig 3: An enzyme molecule binds to the substrate forming a complex called the enzyme substrate complex, on completion of the reactionthe enzyme is released after the product is formed, the enzyme is not altered in the reaction and can even be recycled to break down into additional substrate molecules.
This, thereby makes a substrate more susceptible to certain reactions. Numerous changes such as this take place, the enzyme is released after the product is formed.
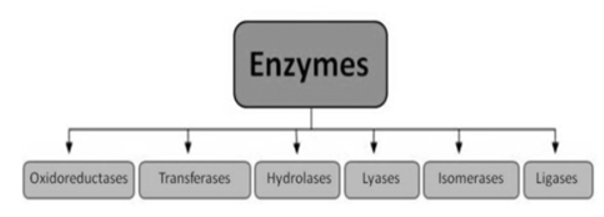
1). Oxidoreductases: These enzymes catalyse the transfer of oxygen or hydrogen atom or electrons from one substrate to the other. (Redox reactions).
E.g., Oxidases, Dehydrogenases, Oxygenises, Peroxidases, Catalases.
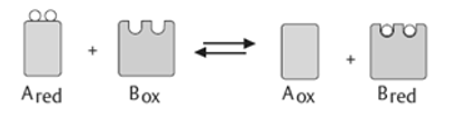
2). Transferases: The transfer of a functional group from one substrate to another is catalysed by this enzyme.
E.g., Kinases, Transaminase.
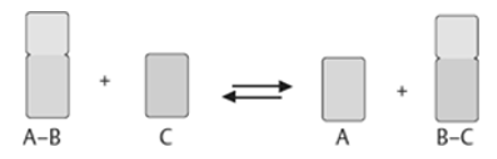
3) Hydrolases: these enzymes catalyse the hydrolysis or breakdown of the substrate.
E.g., Lysozyme, digestive enzymes, acid phosphatase.
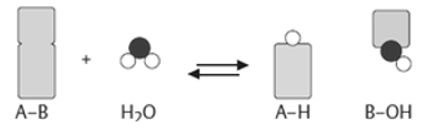
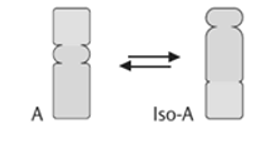
4). Isomerases: they catalyse intramolecular changes in the substrate.
E.g., Isomerase, Fumarase.
5). Lyases: catalyses the non-hydrolytic removal of a group or addition of a group to a substrate.
E.g., Decarboxylases, Aldolases.
6). Ligases (Synthetases): catalyses the joining of two molecules by forming new bonds.
E.g., Citric acid synthetase.
Thereby facilitating the flow of the reaction.
Types | Biochemical Property |
Oxidoreductases | The enzyme Oxidoreductase catalyses the oxidation reaction in this reaction the electrons tend to travel from one form of a molecule to the other. |
Transferases | Functional groups such as donor and acceptor molecules, are transported with the help of enzyme Transferases enzymes. |
Hydrolases | Hydrolases are hydrolytic enzymes, which catalyse the hydrolysis reaction by adding water to cleave the bond and hydrolyse it. |
Lyases | These enzymes eliminate water, carbon dioxide or ammonia or create double bonds or add water, carbon dioxide or ammonia across double bonds. |
Isomerases | The Isomerases enzymes catalyse the structural shifts present in a molecule, thus causing the change in the shape of the molecule. |
Ligases | The Ligases enzymes are known to charge the catalysis of a ligation process. |
An enzyme attracts substrates to its active site, and catalyses the chemical reaction by duringwhich products are formed, and then the products that are formed dissociate (separate from the enzyme surface). The combination formed by the substrate and its enzyme called the enzyme–substrate complex. When two substrates and one enzyme are involved in a reaction, the complex is called a ternary complex; A binary complex is formed when one substrate and one enzyme are involved in a reaction. The substrates are attracted to the active site by hydrophobic and electrostaticand forces, which are called noncovalent bonds because they are physical attractions and not chemical bonds.
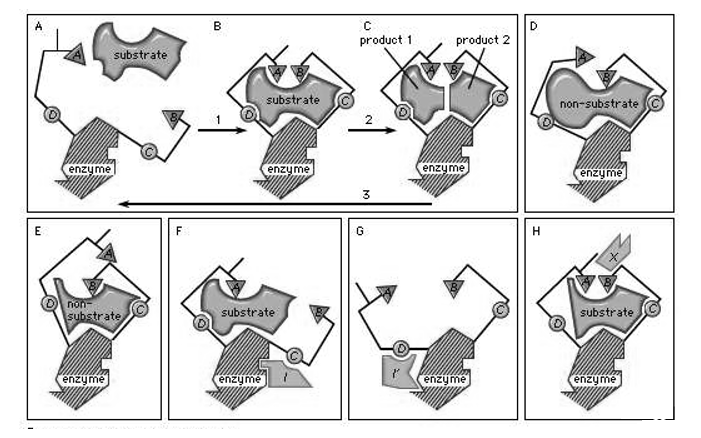
Fig 4: In the induced-fit theory of enzyme-substrate binding, in the first step, a substrate approaches the surface of an enzyme as seen in boxes A, B, C,this reaction causes a change in the enzyme shape that results in the correct alignment of the catalytic groups shown as triangles A and B; the circles C and D in the box represent substrate-binding groups that are present on the enzyme that are essential for catalytic activity. In step 2 the catalytic groups A and B react with the substrate to form endproducts. In step 3 the products formed separate itself from the enzyme thus making the enzyme available to repeat the process. The reactions that occur in boxes D and E show examples of substratemolecules that are too large or very small for proper catalytic alignment. Boxes F and G demonstrate binding of an inhibitor molecule (I and I′) to an allosteric site, thereby preventing interaction of the enzyme with the substrate. Box H illustrates binding of an allosteric activator (X), a non-substrate molecule capable of reacting with the enzyme.
The lock-and-key model refers to the way similar to a lock and key mechanism, as each key is specific to particular lock, in this model also, a substrate binds to an enzyme's active site. They look the samelike a key has to .be specific one for a lock, no reaction takes place if an incorrect substrate tries to bind to a different enzyme.
The active site present in an enzyme is a specific region that receives the substrate. It possesses a unique shape that complements that of the substrate, allowing for specificity to only one or two compounds. The substrate binds to the active site of the enzyme with a particular shape, and a reaction takes place that ultimately causes the release of the product formed. Enzymes catalyse this reaction by easing chemical bond changes in the substrate by altering the distribution of electrons. Once the product has been released, the enzyme regenerates itself and is, ready for another reaction cycle.The lock-and-key analogy sees this process as very specific process, further only a particular key can fit into the keyhole of the specific lock. If the key is in any waya little bigger or smaller, or entirely a different shape, then it does not fit into the specific keyhole, and subsequentlythe reaction cannot occur. The theory was first described by Emile Fischer(lock-and-key analogy) in 1894, and since then many other theories to were discoveredto explain the mechanics of enzyme reactions.
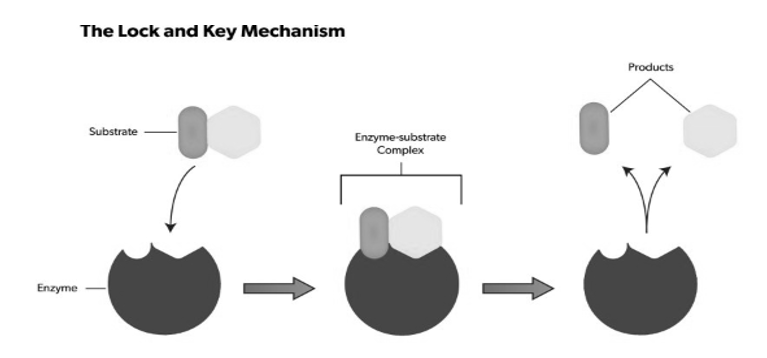
Fig 5: The substrate binds to the active site, and a reaction takes place that ultimately causes the release of the formed product. Enzymes catalyse this reaction by facilitating chemical bond changes in the substrate through altering the distribution of electrons.
The study of the rate at which an enzyme works is called enzyme kinetics. Enzyme kinetics as a function of the concentration of substrate available to the enzyme is observed here.
- A series of tubes is set up containing graded concentrations of substrate, [S].
- To the tubes a specific amount of enzyme preparation at time zero is added.
- The concentration of the product is measure, over the next few minutes. If the product absorbs light, it can be easily done in a spectrophotometer.
- Early in the process, when compared to the enzyme the amount of substrate is in substantial excess, the rate we observe is the initial velocity of denoted as Vi.
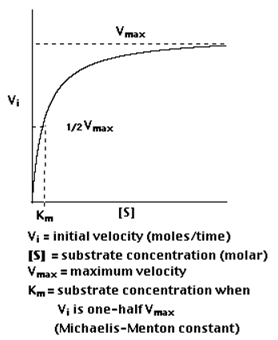
Plotting Vi as a function of [S], the following is observed
- At low values of [S], the initial velocity, VI, rises linearly with increasing [S].
- But as [S] shows an increase, the gains in Vi level shows a drop off (forming a rectangular hyperbola).
- Vmax is designated as the asymptote that represents the maximum velocity of the reaction,
- The substrate concentration that produces a Vi that is one-half of Vmax is designated bythe Michaelis-Menten constant, Km (named after the scientists who developed the study of enzyme kinetics).
Km is roughly defined as the measure which is inverse to the strength or affinity of binding between the enzyme and its substrate. The lower the Km, the greater will be the affinity (so the lower the concentration of substrate needed to achieve a given rate of reaction).
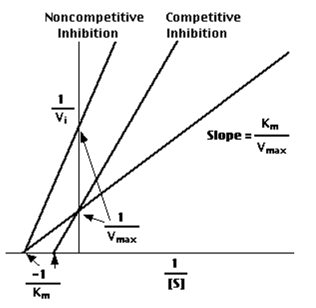
a "double-reciprocal" or Lineweaver-Burk plot is obtained byplotting the reciprocals of the same data points. Vmax and Km is determined precisely by this way which shows accuracy.
- [S] is considered as infinite, Vmax is determined by the point where the line crosses the 1/Vi = 0 axis.
- The magnitude represented by the data points in this plot decrease from lower left to upper right is noticed.
- Km equals Vmax times the slope of line. This is easily determined from the intercept on the X axis.
The Effects of Enzyme Inhibitors
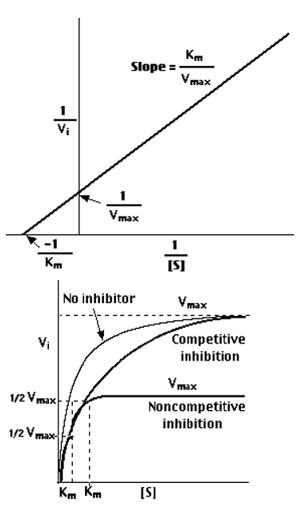
Enzymes can be inhibited in various ways
- Competitively, in this case the substrate and inhibitor compete for binding to the same active site or
- Noncompetitively, this reaction occurs when the inhibitor binds to the enzyme on another location on the enzyme molecule thus reducing the efficiency of enzyme activity.
The distinction can be determined by plotting enzyme activity with and without the presence of an inhibitor.
Competitive Inhibition
In such cases the substrate concentration should be very high if a competitive inhibitor is present, to obtain the same velocity that was present in its absence in the presence of a competitive inhibitor, while Vmax can still be reached if the available substrate concentration is sufficient, however one-half Vmax requires a higher [S] than earlier and thus Km is larger.
Non-competitive Inhibition
With non-competitive inhibition, enzyme molecules that have been bound by the inhibitor are not taken into consideration.
- Enzyme rate (velocity) is reduced for all values of [S], including
- Vmax and one-half Vmax but
- When the enzyme molecules active sites have not been inhibited Km remains unchanged.
Enzymes are protein catalysts that, like all catalysts, speed up the rate of a chemical reaction without being themselves used up in the process.
They achieve their effect by temporarily binding to the substrate and, thereby, lowerthe activation energy needed to convert it to a product.
The rate at which an enzyme works is influenced by many important factors, e.g.,
- The concentration of substrate molecules-
When their availability is more, the quicker the enzyme molecules collide and bind with them). The concentration of substrate is designated [S] and is expressed in units of molarity.
- The temperature-.
As the temperature rises, molecular motion also increases — and therefore collisions between enzyme and substrate — speed up. But as enzymes are proteins, there is an upper limit beyond which the enzyme becomes denatured and ineffective high temperatures can denature proteins.
- The presence of inhibitors.
- Competitive inhibitors are molecules that bind to the same site as the substrate — preventing the substrate from binding to the enzyme active site — but are not changed by the enzyme.
- Non-competitive inhibitors are molecules that bind to some other site on the enzyme reducing the power of the catalysis.
- PH.
PH influences the conformation of a protein and as enzyme activity is crucially dependent on protein conformation, its activity is affected accordingly.
Ribosomes are macromolecular machines found within all living cells, biological protein synthesis is performed by ribosomes (mRNA translation). Ribosomes link amino acids together in a specified order by the codons of messenger RNA(mRNA) molecules leading to the formation of polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA (rRNA) molecules and many ribosomal proteins (RPs or r-proteins). The ribosomes and associated molecules are also known as the translational apparatus
A ribosome is made from complexes of RNAs and proteins and is therefore a ribonuclear protein complex is a complex structure. Each ribosome is divided into two subunits:
- A smaller subunit which binds to a larger subunit and the mRNA, and
- A larger subunit which binds to the aminoacylated tRNAs and the smaller subunit.
The larger and smaller subunits split apart, when a ribosome finishes reading an mRNA molecule. Ribosomes are ribozymes, because the catalytic peptyl transferase activity that links amino acids together is performed by the ribosomal RNA. The rough Endoplasmic reticulum Ribosomes present in the three-domain system namely the bacteria, Archaea and eukaryotes that have a common origin and a lot of similarities are often associated with the ribosomal RNA They differ in their structure, size, sequence, and the ratio of protein to RNA. The differences in structure ofthese cells can permit some antibiotics to kill bacteria by inhibiting their ribosomes, however human ribosomes remain unaffected. However, in few cases one or more than one ribosome moves along a single mRNAat one time also known as polysome. Which is found in all species, each "reading" its sequence and producing a corresponding protein molecule.
The mitochondrial ribosomes present in eukaryotic cells functions resemble many features of those in bacteria, reflecting the likely evolutionary origin of mitochondria.
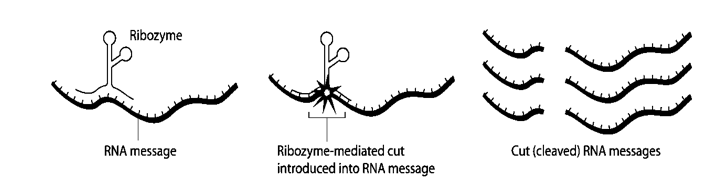
Fig 6: schematic showing ribosome cleavage of RNA
References:
- Enzymes (Biochemistry, biotechnology, clinical chemistry) – Treror Palmer Philiph Bonner
- Catalyst – S J Kincaid
- Enzyme and Enzyme activity – Eva.M.Lashinski