Unit - 8
Metabolism
In physics, energy is considered the quantitative property that is transferred to an object in order to perform work on, or to heat, the object. Energy is a conserved form or quantity; Energy can neither be destroyed or created that complies to the law of conservation of energy. The SI Unit of energy is the joule.
The other common forms of energy include the kinetic energy is the energy required to move an object, in a field with force involved the potential energy stored by an object's position can be (gravitational, electric or magnetic), when solid objects are stretched the elastic energy is visible in them, the chemical energy released when a fuel burns, and the thermal energy due to an object's temperature and the radiant energy carried by light.
However, in case of Mass and energy they are closely related. Due to mass energy equivalence, when an object is stationary it has a mass (called rest mass) also has an equivalent amount of energy whose form is called rest energy, and any additional energy (of any form) when acquired by the object than the rest energy will result in an increase the object's total mass just as it increases its total energy. For example, after heating an object, there is an increase in energy that could be measured as a small increase in mass, with a sensitive scale.
Living organisms require energy to survive, one such energy is the energy humans get from the food we eat. Human civilization requires energy for all activities, this energy is obtained from sources such as fossil fuels, nuclear fuel, or renewable energy. The processes of Earth's ecosystem and climate and are driven by the radiant energy and the Earth receives natural energy from the sun and the geothermal energy contained within the earth.
Energy can be obtained through different ways. Sun is obviously the biggest energy source but there are few chemical reactions organisms can utilize for their benefit too. Autotrophs the primary producers, are organisms that prepare their own food through photosynthesis or chemosynthesis, they use these energy sources to convert sun light or inorganic chemical energy, respectively, to organic chemical energy.
Eukaryotes do these in their chloroplasts, prokaryotes do these in many different ways due to the diversity of chemosynthesis. Prokaryotes can convert photosynthetically active radiation (PAR), the part of sunlight that can be used in photosynthesis, to chemical energy with mainly chlorophylls. Same molecules that are in the chloroplasts of eukaryotes.
The laws of thermodynamics are important unifying principles of biology. These principles govern the chemical processes (metabolism) in all biological organisms.
All biological organisms require energy to survive. In our universe which is a closed system, such as the universe, here the energy is neither consumed but transformed from one form to another. Cells, for example, require energy for a number of processes they perform
First Law of Thermodynamics in Biological Systems
In photosynthesis, the main source of energy is supplied by the sun. This Light energy is absorbed by cells present in plant leaves and converted to chemical energy. The chemical energy that is obtained is further stored in the form of glucose, which is later utilised to form complex carbohydrates necessary to build plant mass.
The energy that is stored in glucose by plant may be released through a process called cellular respiration. This process allows plant and animal organisms to obtain the energy stored in lipids, carbohydrates, and other macromolecules through the production of ATP. This ATP is necessary and is required to perform cell functions such as DNA replication, Cytosis, mitosis, meiosis, cell movement, exocytosis, and apoptosis.
Second Law of Thermodynamics in Biological Systems
As with other biological processes, there is not complete transfer of energy and sometimes may not be efficient. In photosynthesis, for example, not all of the light from the sun is absorbed by the plant. Some energy gets reflected and some is lost as heat. There will be an increase in disorder or Entropy when the energy lost spreads to the surroundings. Animals cannot generate energy directly from the sun, unlike plants and other photosynthetic organisms. Animals must consume plants or other animal organisms for energy.
As we move higher up in a food chain, the higher up an organism is, the less available energy it receives from its food sources. The reason being much of this energy is lost during metabolic processes performed by the producers and primary consumers that are eaten. Resulting in much less energy is available for organisms at higher trophic levels. (Trophic levels are groups that help ecologists understand the specific role of all living things in the ecosystem.) The lower the available energy, the smaller number of organisms can be supported. This is why there are more producers than consumers in an ecosystem.
The Living systems require constant input ofenergy to maintain their highly ordered state. Cells, for example, are highly ordered and have low entropy. Some energy is lost or transformed in the process of maintaining this order. So, while cells are ordered, the processes performed to maintain that order result in an increase in entropy in the cell's/organism's surroundings. The transfer of energy causes entropy in the universe to increase.
Endothermic Reactions
The endothermic process, are reactions that take place when the system absorbs energy from its surroundings in the form of heat. Few examples for the endothermic process are ammonium chloride, dry ice, alkanes cracking, photosynthesis, evaporating liquids, melting ice, thermal decomposition, ammonium chloride in water and much more.
Exothermic Reactions
The exothermic reaction is the reverse reaction that occurs in an endothermic reaction. In this process the system releases energy by light or heat to its surrounding. Few examples of this reaction deposition of dry ice, neutralization, burning a substance, respiration, reactions of fuels, deposition of dry ice, solution of sulfuric acid into water
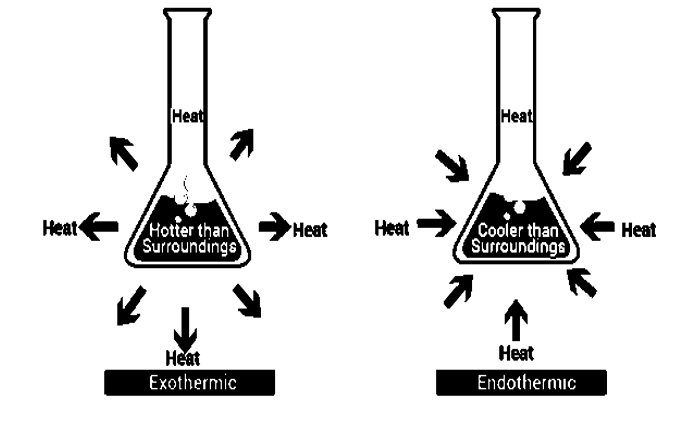
Fig 1: The differences that occur in both reactions, in an Endothermic reaction heat is absorbed into the system, where as in exothermic reactions heat is given out from the system.
Endergonic and Exergonic Reactions
The word “ender” has derived from the word “endo” meaning from “within”. Therefore, endergonic means these reactions absorb energy in the form of work. Therefore, in an endergonic reaction, the surrounding environment supplies energy into the system. Further, the products formed will have a higher energy than the reactants. Endergonic reaction is considered to be nonspontaneous or unfavourable. Gibbs free energy will be positive if this energy transfer takes place in a constant pressure and temperature. Thus, for endergonic reactions the equilibrium constant is less than one. The best example for this reaction is Photosynthesis which takes places in the natural environment. The energy required for photo synthesis supplied by sunlight. Where as in the human body, when endergonic reactions are taking place, the energy is supplied most of the time by ATP. So, in many cases the Endergonic reactions are coupled to ATP hydrolysis reactions.
Exergonic means releasing energy in the form of work. In these reactions, energy is released to the outside from the system. Exergonic reactions are favourable and spontaneous. In this case the products possess less energy than the reactants because the energy is released during the reaction. Therefore, the enthalpy change (∆H) becomes negative. Moreover, the Standard Gibbs free energy will be negative, if the transfer is carried out in constant pressure and temperature.
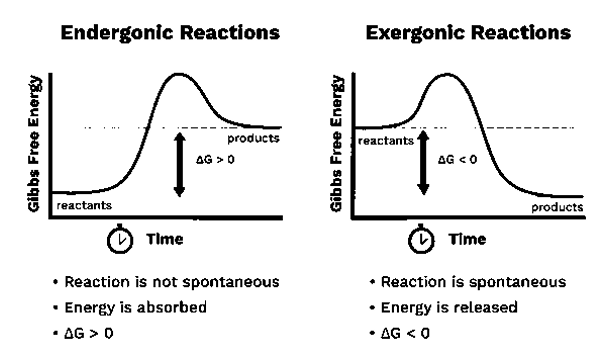
Fig 2: The difference between endergonic and exergonic reactions.
The free energy change of the reaction in any state, ΔG (when equilibrium is not yet reached) of a reaction is related to the standard free energy change that takes place in a reaction, ΔG0 ( In a standard state both the differences in free energy of the reactants and formation of products will be equal) according to the equation.
ΔG = ΔGo + RT InQ
Q is the reaction quotient
There is no free energy change when equilibrium is reached i.e. ΔG = 0 and Q becomes equal to equilibrium constant. Hence the above equation becomes.
ΔGo = –RT in K(eq)
Or ΔGo = –2.303 RT log K(eq)
In the case of galvanic cells. The electrical work done by the cell is related to Gibbs energy change ΔG.
ΔG = -nFE(cell) where n = no. Of moles of electrons involved
F = the Faraday constant
E = emf of the cell
If reactants and products are in their standard states ΔGo = –nFEocell
Glycolysis is the anaerobic reaction that involves the catabolism of glucose. This process occurs in the cytosol. As its name implies, the pathway uses several enzyme catalysed reactions to split (lysis) a sugar (glycol).
The Individual Reactions of Glycolysis
The pathway of glycolysis can be seen as consisting of 2 separate phases. The first phase requires energy in the form of ATP for the chemical priming phase, and the second is considered to be the energy-yielding phase. In the first phase, 2 equivalents of ATP are used to convert glucose to fructose 1,6-bisphosphate (F1,6BP). In the second phase F1,6BP is degraded to pyruvate, with the production of 4 equivalents of ATP and 2 equivalents of NADH.
GLYCOLOSYS YIELDS: 8 ATP; 2NADH + H+, 4 ATP, -2 ATP
C6H12O6 +2NAD+ ->2C3H4O3+2NADH+2H+
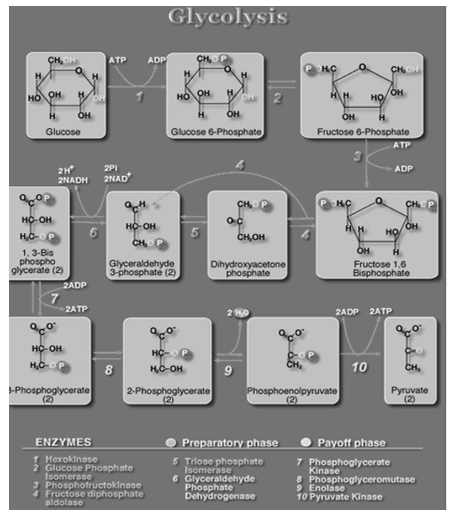
Fig: 3 The steps involved in glycolysis
The Krebs cycles
In the glycolysis cycle pyruvate molecules are produced and they contain a lot of energy in the bonds present between their molecules. The pyruvate molecules are processed through the krebs cycle to convert the energy into ATP so that it is used in all cell activities. Krebs cycle, is also called as the Citric acid cycle. The krebs cycle occurs in the Mitochondria of the cell.
Before entering the Krebs cycle, pyruvate has to be converted into acetyl CoA. This is
Achieved by the removal of a CO2 molecule from pyruvate and then removing an electro to reduce an NAD+ into NADH. An enzyme called coenzyme A combines with the remaining acetyl to make acetyl CoA, after which it enters the Krebs cycle.
The steps in the krebs cycle are summarized below:
- Citrate is formed when acetyl group from acetyl CoA combines with oxaloacetate
- Citrate is converted to its isomer, isocitrate
- Isocitrate is oxidised to form the 5-carbon α-Ketoglutarate. In this NAD+ is reduced to NADH2+and releases one molecule of CO2.
- The α-Ketoglutarate is oxidised to succinyl CoA yielding CO2andNADH2+
- Succinyl CoA releases coenzyme A and Phosphorylates ADP into ATP.
- Succinate is oxidised to fumarate, converting FAD to FADH2.
- Fumarate is then hydrolysed to form Malate.
- Malate is oxidised to oxaloacetate, reducing NAD+ to NADH2+
In the Krebs cycle, during the glycolysis process to pyruvate molecules from one glucose, each glucose is processed through the Krebs cycle two times. For each molecule of glucose ,6 NADH2+,2FADH2 and 2 ATP are formed.
Krebs cycle yields (2 cycles,1 for each pyruvate):24 ATP 6 NADH +h ,2 GTP, 2FADH.
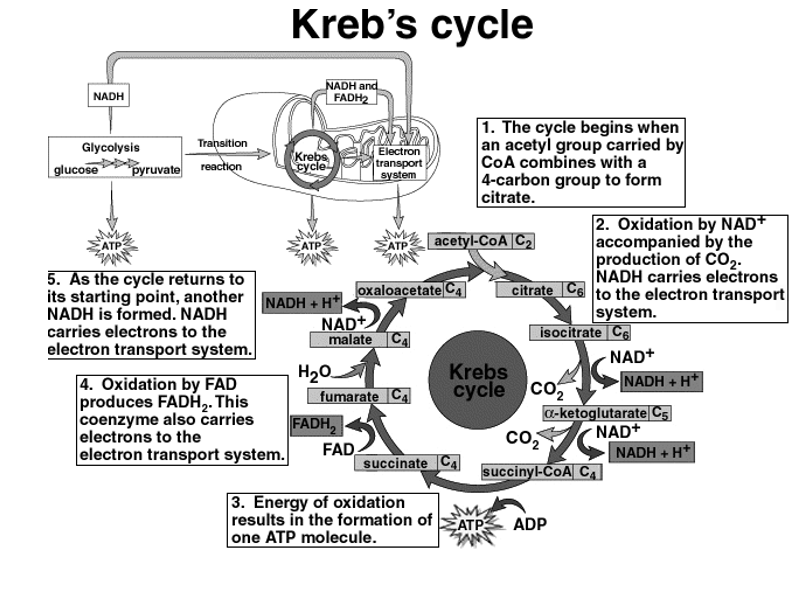
Fig 4: Overview of Krebs Cycle
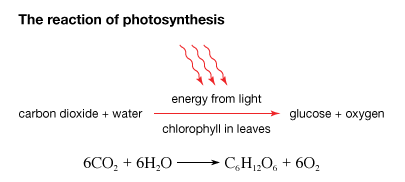
Photosynthesis is an important process involving a biochemical pathway that results in the production of sugar (glucose) from sunlight, water and carbon dioxide and in the process releases oxygen. Photosynthesis occurs in few bacteria, algae, higher plants and some photoautotrophs. It occurs in a series of complex bio chemical reactions. Nearly all life depends on this process. The rate of photosynthesis is directly related to concentration of carbon dioxide, temperature and light intensity. It gets energy from absorbed photons present on the leaves and uses water as a reducing agent.
Carbohydrates are involved in many essential metabolic pathways. Plants utilize sunlight and synthesize carbohydrates from carbon dioxide and water through photosynthesis, energy is stored in the form of carbohydrates through this sunlight internally. In cellular respiration the stored carbohydrate is broken down to release energy by animals and fungi that consume plants. Both animals and plants store the released energy temporarily in the form of high-energy molecules, called ATP, for use in various cellular processes.
Photosynthesis is a chemical reaction in which carbon dioxide and water combine to produce glucose and oxygen. Sunlight forms the source of energy, which is absorbed by pigments, called chlorophyll. Chlorophyll is green in colour and is the pigment that gives plants their green colour
Glycolysis
Glycolysis is the process during which glucose molecules are broken down into two pyruvate molecules, while storing energy released during this process as ATP and NADH. Glycolysis is a process utilised by all organisms. Glucose regulation and product formed are the primary categories in which these pathways differ between organisms. In some tissues and organisms, glycolysis is the only method of energy production. This pathway is common to both anaerobic and aerobic respiration.
Glycolysis proceeds in ten steps, split into two phases. During the first phase, two ATP molecules are broken down. During the second phase, chemical energy from the intermediates is transferred into ATP and NADH. The breakdown of one molecule of glucose gives rise to two molecules of pyruvate, which can be further oxidized to access more energy in later processes.
Through feedback regulation Glycolysis can be regulated at different steps of the process. The third step is the most regulated one. This regulation is to ensure that the body is not over-producing pyruvate molecules. The regulation also allows for the storage of glucose molecules into fatty acids. There are various enzymes that are used throughout glycolysis.
Glycolysis
1. Glycolysis takes place in the cytoplasm
2. It can Occur in the presence or absence of oxygen
3. They Involve ten enzyme-catalysed reactions
4. Broken down into 2 molecules of pyruvate
5. Steps
A. Glucose is converted to a 6-C diphosphate. This uses 2 ATP's
B. The 6-C molecule is broken down into 2, 3-C molecules
C. The 2, 3-C sugars are converted to Pyruvate, gaining 4 ATP and 2 NADH
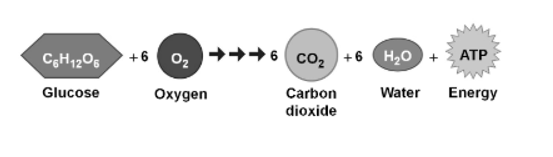
Fig 5: Glycolysis is the process during which glucose molecules are broken down into two pyruvate molecules, while storing energy released during this process as ATP and NADH with water and carbon dioxide.
The processes of breaking down carbohydrate molecules or to build these molecules illustrate two types of metabolic pathways. A metabolic pathway is a step-by-step series biochemical reaction that are interconnected and convert a substrate molecule or molecules into a final product or products through a series of metabolic intermediates. For example, one metabolic pathway involves breaking down of carbohydrates into large molecules of glucose. Another metabolic pathway might build glucose and form large carbohydrate molecules used for storage. The first process requires energy and is referred to as anabolic reaction. The second process on the other hand produces energy and is referred to as catabolic reaction. Consequently, metabolism is composed of these two opposite pathways:
- Anabolism (building molecules)
- Catabolism (breaking down molecules)
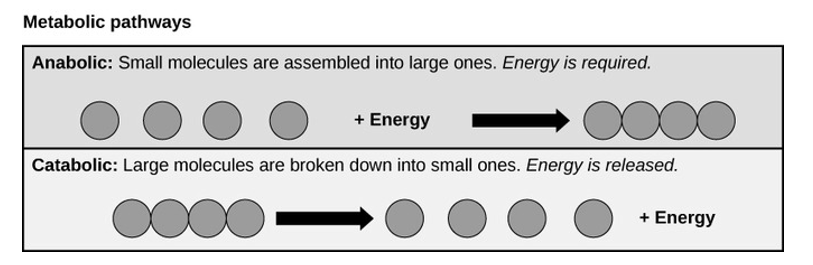
Anabolic and catabolic pathways: Anabolic pathways is a process that require energy to synthesize larger molecules. Catabolic pathways are those that generate energy by breaking down larger molecules. However, both types of pathways are required for maintaining the cell’s energy balance.
Anabolic Pathways
Anabolic pathways require an energy input to synthesize complex molecules from simpler ones. A good example of anabolic pathway is the synthesis of sugar from CO2. Other examples include breaking down of amino acid to form large proteins and the synthesis of new DNA strands from nucleic acid building blocks. These processes are very critical to the life of each cell, and take place constantly, and eventually these processes demand energy provided by ATP molecules and other high-energy molecules like NADH (nicotinamide adenine dinucleotide) and NADPH.
Catabolic Pathways
Catabolic pathways involve the breaking down of complex molecules into simpler ones, releasing the chemical energy is stored in the bonds of those molecules. Some catabolic pathways can capture the chemical energy to produce ATP, which is the energy molecule used to power all cellular processes. Other energy-storing molecules, such as lipids, are also broken down through similar catabolic reactions to release energy and make ATP.
Electron charge, (symbol e), is a fundamental physical constant that is expressed, it is the naturally occurring unit of electric charge, that is equal to 1.602176634 × 10 −19 coulomb. All subatomic particles that exist freely that have been discovered so far have an electric charge equal to this number or some whole-number multiple of it.
Electrons play an essential role in various ways they are part of weak interactions and also participate in electromagnetic and gravitational interactions, they play an essential role in numerous physical phenomena, such as chemistry, conductivity, electricity, thermal conductivity and magnetism, and they also participate in electromagnetic, gravitational, and weak interactions. Since an electron has charge, it has a field surrounding it known as the electric field, a magnetic field will be generated when the electron moves relative to an observer. When the electrons are accelerated or they speed up, the electrons absorb or radiate energy in the form of photons. With the use electromagnetic fields electrons can be trapped individually by laboratory instruments. Electrons are involved in many other applications.
References:
- Biological Thermodynamics-Dr.B.Victor
- The Journey of Glucose Molecules- Shonnah Stephen
- The Biochemistry of Plants; A Comprehensive Treatise- P.K Stumfand E.E Conn.