Unit - 1
Fluid
In simpler terms a fluid is a substance which can flow under the action of shear force (however small force may be). In physics, a fluid is a liquid or gas that continually deforms (flows) under an applied shear stress, or external force. They have zero shear modulus.
Fluids generally include liquids, gases and plasmas. To some extent, plastic solids are also considered fluids.
Example- Liquid, gases, vapour.
For a static fluid, shear force is zero.
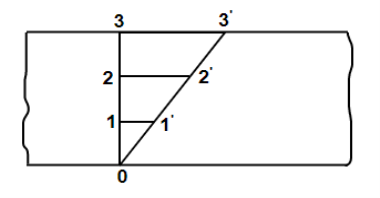
Figure 1: Force at static fluid
Solid regains their original shape if the shear force within elastic limit when the force is removed. But fluid never regains original shape after the removal of force.
Fluids have common properties that they share, such as compressibility, density, pressure, buoyancy and viscosity.
Fluids are basically separated in 5 types
- Ideal fluid
- Real fluid
- Newtonian fluid
- Non –Newtonian fluid
- Ideal plastic fluid
1.ideal fluid –fluid which is incompressible in nature and no viscosity.in practical there is no ideal fluid, because all fluids have viscosity. Also called as imaginary fluid.
2.Real fluid - fluid which is compressible in nature and viscosity
Ex. Kerosene, petrol, castor oil.
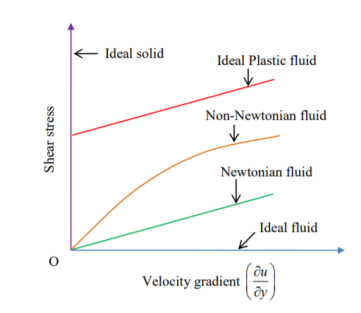
3. Newtonian fluid – fluid which obeys Newton’s law of viscosity. In other words, fluids in which shear stress is directly proportional to rate of shear strain. This fluids viscosity totally depends on temperature and pressure of fluid.
Ex. Water, air, Hydrogen
4. Non –Newtonian fluid- fluid does not obey Newton’s law of viscosity. In other words, fluids in which shear stress is not directly proportional to rate of shear strain.
Ex – Flubber, Ooblek
5. Ideal plastic fluid - words fluids in which shear stress is directly proportional to rate of shear strain and in
Which shear stress is more than yield value.
S.I. Unit of Surface tension is N/m
The dimensional formula of surface tension is MT-2.
The ratio of shear stress to shear rate is a constant, for a given temperature and pressure, and is defined as the viscosity or coefficient of viscosity.
According to Newton’s law of viscosity the shear stress is directly proportional to rate of shear strain or rate of angular deformation or velocity gradient. The fluid which follows this is law is called newtonion fluid.
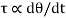
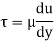
Newton’s law of viscosity is similar to hooke’s law for solid.
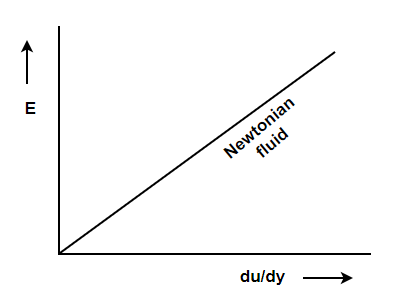
Figure 2: Diagram for newton’s law of viscosity
There are various properties of fluid. Some of them are mentioned below:
- Mass Density
- Specific weight or weight density.
- Specific Gravity
- Compressibility
- Viscosity
It is defined as the ratio of mass of the fluid to its volume. Its unit is kg/m3 and its dimensional formula is ML-3.
Density depends on temperature and pressure.
Specific volume is defined as the ratio of volume of fluid to the mass of fluid.
It is denoted by Vs.
Mathematically it is denoted as
Vs=V/m=1/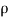
It is defined as the ratio of density of fluid to the density of standard fluid.
Or
It can be defined as the ratio of specific weight of fluid to the specific weight of standard fluid.
It is unit less or dimensionless.
For liquid, the standard fluid is water and for gases the standard fluid is either H2
Or air at given temperature and pressure.
The specific gravity of water is 1.
If the specific gravity of liquid is less than 1 that means it is lighter than water and if the specific gravity of liquid is greater than 1, it means the liquid is heavier than water.
It is the internal resistance offered by one layer fluid to the other layer.
Viscosity is the physic al property that characterizes the flow resistance of simple fluids.
Viscosity is the property of a fluid by virtue of its offer’s resistance to the movement of one layer of fluid over an adjacent layer.
Viscosity is also called as dynamic viscosity
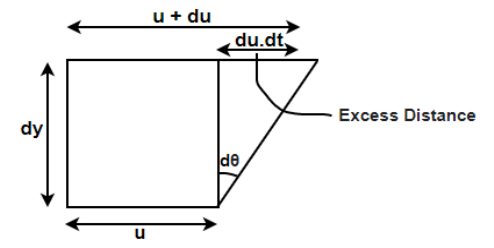
Figure 3: Viscosity diagram
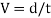
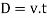
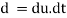
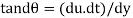
As the tandθ is very less, then
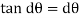
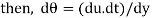
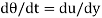
We know that, shear stress= shear force/shear area
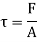
As the molecules are taken into same area:
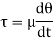
Kinematic viscosity
It is defined as the ratio of dynamic viscosity to the density of the fluid.it is also called as momentum diffusivity:
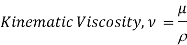
Where
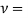 Kinmeatic viscosity
Kinmeatic viscosity
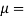 Dynamic Visocosity
Dynamic Visocosity
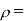 Density of the fluid
Density of the fluid
“Surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise surface area”.
Surface tension is a property of liquids that arises from unbalanced molecular cohesive forces at or near a surface
Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. Surface tension allows insects (e.g., water striders), to float and slide on a water surface without becoming even partly submerged.
At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion).
There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane.
Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface tension (72.8 milli newtons (mN) per meter at 20 °C) than most other liquids. Surface tension is an important factor in the phenomenon of capillarity.
Surface tension has the dimension of force per unit length, or of energy per unit area. The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids.
In materials science, surface tension is used for either surface stress or surface energy.
Formula for surface tension
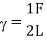
Where, 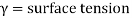
L=Length
F= force
S.I. Unit of Surface tension is N/m
The dimensional formula of surface tension is MT-2.
Examples of surface tension in action include the following:
--formation of liquid droplets,
--the ability of a needle to float on water,
--why bubbles are round
--soap being used the break up water tension.
Capillarity:
It is the rise or depression of a liquid in a small passage such as a tube of small cross-sectional area, like the spaces between the fibres of a towel or the openings in a porous material.
Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to right) is limited by surface tension and, of course, gravity.
Capillary action occurs because water molecules bond each other strongly due to forces of cohesion and adhesion where water molecules are attracted and stick to other substances such as glass or paper.
Adhesion of water to the surface of a material will cause an upward force on the liquid.
The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the surface material is stronger than the cohesive forces between the water molecules.
The height to which capillary action will take water is limited by surface tension and gravity.
A tube of very fine bore is called capillary. The phenomenon of rise or fall of a liquid in a capillary tube is called capillarity.
If we consider meniscus to be hemispherical in shape, the weight of the liquid rise (the total upward force) = (Volume of liquid in cylinder up to h + volume of liquid in cylinder of radius r and height r – volume of hemisphere of radius r) ρg.
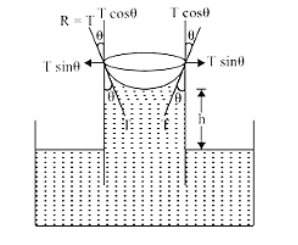
Figure 4: Capillarity phenomenon
2πr×Tcosθ=[πr2×h+πr2×r−23πr3] ρg2πr×Tcosθ=[πr2×h+πr2×r-23πr3] ρg
T=r(h+r3) ρg2cosθT=r(h+r3) ρg2cosθ.
For capillary r3r3 can be neglected h=2Tcosθrρgh= 2Tcosθrρg.
This is called Ascent formula.
Key Takeaways:
It is the rise or depression of a liquid in a small passage such as a tube of small cross-sectional area, like the spaces between the fibres of a towel or the openings in a porous material.
Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature. The temperature at which the vapour pressure at the surface of a liquid becomes equal to the pressure exerted by the surroundings is called the boiling point of the liquid.
To find the vapour pressure at a given temperature,
Use the Clausius- Clapeyron equation:
Ln(P1/P2) = (ΔHvap/R) ((1/T2) - (1/T1)).
You could also use Raoult's Law to find the vapor pressure:
P solution = X solvent P solvent
P solution=vapor pressure of the solution
X solvent=mole fraction of the solvent
Psolvent=vapor pressure of the pure solvent
Key Takeaways:
Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature.
The relation between compressibility and bulk modulus is that the inverse of compressibility is known as the bulk modulus.
Bulk modulus is defined as the ratio between increased pressure and decreased volume of the material.
Bulk Modulus is defined as the ratio of compressive stress to volumetric strain.
K= 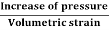
Compressibility is defined as the ratio of change in volume to the change in pressure. It is reciprocal of Bulk modulus (K)
ß = 1/K
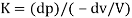
-ve sign means the volume decrease with increase in pressure.
We know that, 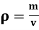
Since, R= Characteristics gas constant and T is constant (isothermal process).
Differentiating the above equation
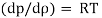
Now the isothermal bulk modulus
KT=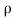 .(dp/d
.(dp/d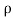 )=
)= 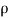 . RT=P
. RT=P
KT=P
Manometers, buoyancy, uniformly accelerated motion.
The hydrostatic pressure is the pressure exerted by a fluid at equilibrium at any point of time due to the force of gravity. Hydrostatic pressure is proportional to the depth measured from the surface as the weight of the fluid increases when a downward force is exerted.
Fluids exert pressure in all directions equally. This rule leads to another interesting occurrence. If we consider the layer of water on the top of a bottle, the pressure exerted by the layer of water acts on the container at the sides, the surface of air on top and the layer of water at the bottom. As we move down from the top of the bottle to the bottom, the pressure exerted by the top layer on the bottom adds up.
Due to this phenomenon, the fluid at the bottom of the container experiences more pressure that the fluid which is above it.
Hydrostatic force refers to the total pressure acting on the layer or surface which is in touch with the liquid or water at rest. If the liquid is at rest, then there is no tangential force, and hence the total pressure will act perpendicular to the surface with contact.
The location of total pressure is referred as the centre of pressure which is always below the center of gravity of the surface in contact.
1.12.1 Fluid force on plane and curved surface:
Forces on the curved surface
For forces on the curved surface, there will be two forces required to determine the resultant hydrostatic force.
• Horizontal force
• Vertical force
Horizontal force on curved surface
The vertical plane shall be considered to determine the horizontal force, which is the vertical projection of the curved surface generally rectangle. But in case of hemispherical or spherical, it becomes circular shape.
Express the horizontal component of force.
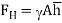
Vertical force on curved surface
It is the weight of the liquid acting on the curved surface in contact with the liquid which may be in upward direction due to buoyancy or downward direction due to the weight of the fluid.
Express the vertical component of force.
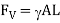
Therefore, the resultant force on the curved surface is,
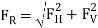
1.12.2 Manometers:
- Manometer is a simple and inexpensive device of measuring pressure and pressure difference.
- It is usually bent to form a U-tube and filled with liquid of known specific gravity. The surface of the liquid will move in proportion to changes of pressure.
- A manometer is one of the most accurate devices for measuring pressure in the lower ranges.
- Since manometers are so accurate, they are often used as calibration standards.
Types of Manometers:
1. Piezometer:
- Piezometer is the simplest form of simple manometer which is used to measure the gauge pressure of a fluid in a container by using the concept of hydrostatic pressure.
- Piezometer is only suitable to measure the pressure of fluid in a container, if pressure in the container is more than the atmospheric pressure i.e., Gauge pressure.
- Though effective in many purposes, piezometer is not practical to use in lighter liquids with large pressure and cannot be used to measure gas pressure.
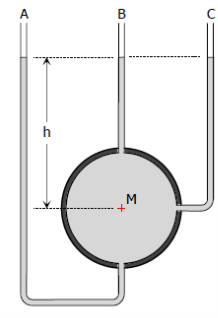
Figure 5: Piezometer
- From the figure above, three piezometers A, B, and C are attached to a pressure conduit at bottom, top, and side, respectively. The column of liquid at A, B, and C will rise at the same level above M indicating a positive pressure at M.
- Rise of liquid in the glass tube of Piezometer will provide us the pressure head at point A and could be written as mentioned here. Rise of liquid in the glass tube of Piezometer will also be termed as Piezometric head.
Gauge pressure at point A = ρ x g x h
Where,
ρ = Density of liquid
g = Acceleration due to gravity
h = Rise of liquid in Piezometer glass tube
Absolute pressure at Point A = Pa + ρ x g x h
Where, Pa is the atmospheric pressure
2. Open Manometer:
Open manometer is a tube bent into a U-shape to contain one or more fluids of different specific gravities. It is used to measure pressure. Example of open manometer is shown below.
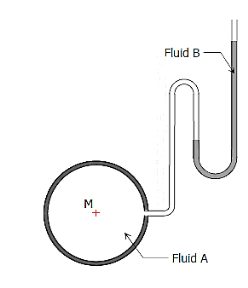
Figure 6: Open Manometer
3. Differential Manometer:
Differential manometer cannot measure pressure but can measure pressure difference. Frequently in hydraulic problems, difference in pressure is more useful information than the pressure itself.
Figure 7: Differential Manometer
1.12.3 Buoyancy:
- Buoyancy is the force that causes objects to float. It is the force exerted on an object that is partly or wholly immersed in a fluid. Buoyancy is caused by the differences in pressure acting on opposite sides of an object immersed in a static fluid. It is also known as the buoyant force. OR
- The upward force applied by the fluid on the object or the body when an object is put in or submerged in the fluid.
- The unit of the buoyant force is the Newton (N).
Force of Buoyancy:
- When we submerge an object in a fluid, an upward force is experienced by the object. This force is applied by the fluid on the objects which makes it to, rises up and is called the Force of Buoyancy.
- The scale of this force is precisely equal to the amount or weight of the liquid displaced.
- The buoyant force depends on (1) The volume of the body immersed which is equal to the volume of fluid displaced. (2) The density of the fluid.
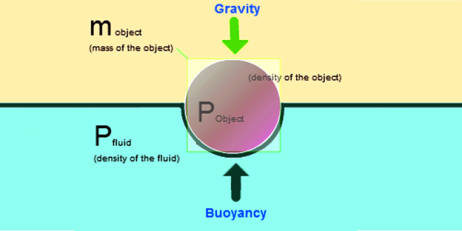
According to the Archimedes’ Principle
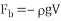
Fb=buoyant force
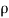 =fluid density
=fluid density
g=acceleration due to gravity
V=fluid volume
The unit of the buoyant force is the Newton (N).
Center of Buoyancy:
The point where the force of Buoyancy is applied or the point on the object where the force acts are termed as the Center of Buoyancy.
It should be illustrated that the force of buoyancy is a vertical force, and thus, the Center of Buoyancy is the point situated on the centre of the gravity of the liquid that is being displaced by the object submerged.
1.12.4 Uniformly Accelerated motion:
In general, a uniformly accelerated motion is the one in which the acceleration of the particle throughout the motion is uniform. It can be moved in one dimension, two dimensions, or three dimensions.
If an object's speed (velocity) is increasing at a constant rate then we say it has uniform acceleration. The rate of acceleration is constant. If a car speeds up then slows down then speeds up it doesn't have uniform acceleration.
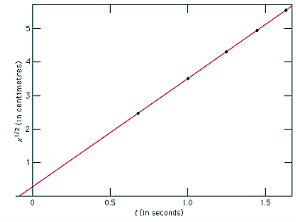
Figure 8: uniform Acceleration motion
Key takeaways:
In general, a uniformly accelerated motion is the one in which the acceleration of the particle throughout the motion is uniform. It can be moved in one dimension, two dimensions, or three dimensions.
References:
- Frank M. White, Fluid mechanics, Tata McGraw Hills.
- Som and Biswas, Fluid mechanics and machinery.
- Various presentations and pdf from various websites.