Unit 1
Fundamentals
BASIC CONCEPTS OF THERMODYNAMICS
Thermodynamics is a branch of science that deals with various forms of energy and its transformation from one form to another.
Thermodynamics deals with the behaviour of gases when subjected to variations of pressure and temperature and the relation between heat and mechanical energy or work.
When a substance changes from one condition to another in a process, energy transformation may occur. Common processes are heating, cooling, expansion, compression.
Let us discuss basic definitions that we have already studied in Physics.
Working Substance or Medium
Thermodynamic process requires a carrier that would act as mode of transport of energy from or into the system. Such a medium is called as working substance or working medium.
For example, petrol is the working medium in SI engines whereas diesel is the working medium in CI engines.
System
Thermodynamic system is the region which is under observation for thermodynamic changes.
There are 3 types of systems thermodynamics
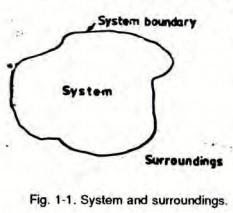
- Isolated systems,
- Closed system, and
- Open system
An isolated system cannot exchange either mass or energy with the surroundings.
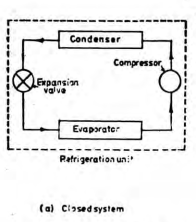
A closed system exchanges energy with the surrounding but mass transfer is not possible.
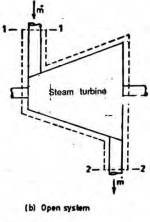
An open system can exchange both mass and energy with the surroundings.
State and Properties of a Substance
The exact condition of a substance is called its state.
Variables which determine the state of a substance are called its properties or parameters.
Pressure, temperature, volume, internal energy, enthalpy, entropy, heat are the properties that can be used to accurately place the exact condition of the substance. Minimum two of these of these are required to pin point the state of any substance.
Process and Cycle
The path followed by some property of substance when it undergoes changes, it is said to have undergone a process.
Process is named according to its specification, i.e., constant pressure process, constant volume process, etc.
A cycle is a combination of processes so that the initial and final states of the system are the same. A thermodynamic cycle is also known as a cyclic operation of processes.
Energy:
Energy may be defined as the capacity of a body to perform work. It is of many forms and can be converted from one form to another. Energy is a path function that is it depends on the path followed by the process and not on the end states.
Exact differentials:
The properties that are expressed at a particular state of the system and are not at all dependent on the path followed by the system are called path functions. Examples are pressure, temperature, volume, etc.
Exact differentials are the derivatives of some other functions as the name suggests.
These are used to express change in point functions. Examples are pressure change dP, volume change dV, etc.
Inexact differentials:
The properties that depend on the path followed by system and not on the state of the system are called path functions. Examples are work, heat.
Inexact differentials are useful for expressing very minute quantities in these path functions.
Work:
Work is a transient form of energy. Work is done when the application of some form of force results in change in state of the body. The unit of work is newton-metre (N-m), which is the product of a unit force (one newton) and unit distance (one metre) moved in the direction the force.
This unit of work is also known as joule (J),
that is 1 joule = 1 newton-metre (N-m)
In thermodynamics, work done by a system, is taken to be positive, and when work is done on a system it is taken to be negative.
Displacement Work:
Consider a piston sliding inside the cylinder as shown in the figure.
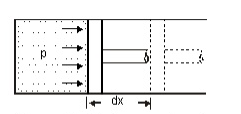
The pressure is being exerted on the piston by the expanding fluid which causes the piston to displace towards right.
Force acting on the piston, F = pressure, p x area, A
= pA
Work done in displacement = Force x displacement
W = pA x dx
= pdV
This work done is always dependent and changes according to the path followed by the system.
This is illustrated by simple processes given below.
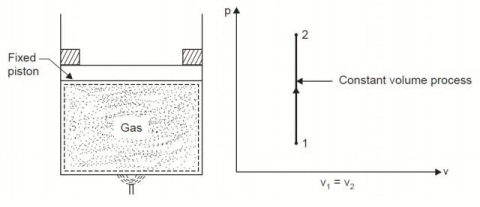
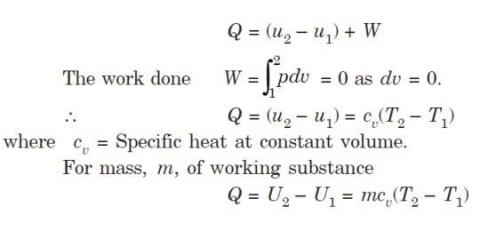
Considering mass of the working substance unity and applying first law of thermodynamics to the process,
2. Constant Pressure or Isobaric Process:
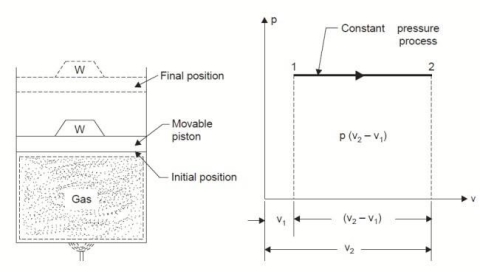
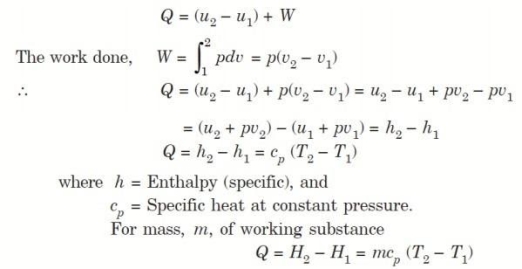
Considering unit mass of working substance and applying first law of thermodynamics to the process,
3. Constant Temperature or Isothermal Process:
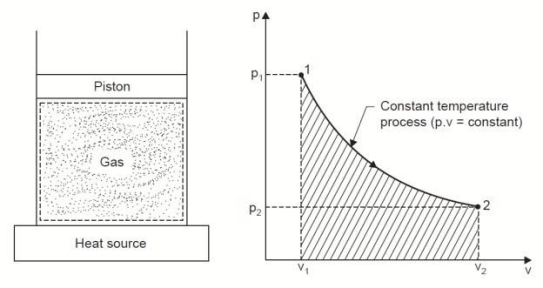
Considering unit mass of working substance and applying first law to the process,
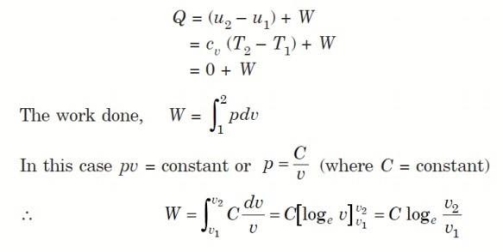
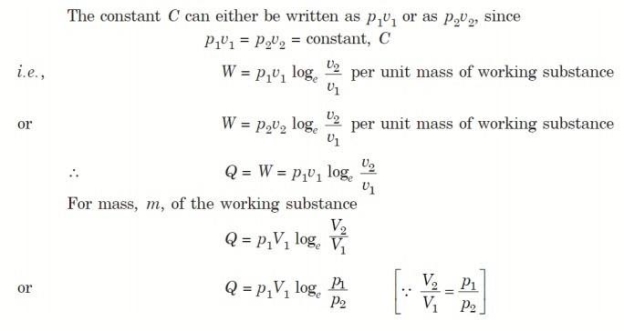
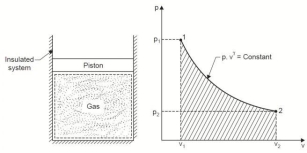
4. Reversible Adiabatic Process (PV γ = constant)
An adiabatic process is one in which no heat is transferred to or from the fluid during the process. Such a process can be reversible or irreversible.
Considering unit mass of working substance and applying first law to the process
Q = (u2 – u1) + W
O = (u2 – u1) + W
or W = (u1 – u2) for any adiabatic process
Above equation is true for an adiabatic process whether process is reversible or not.

Electrical Work:
Electrical work is defined as the work required to move an unit charge once around the complete circuit.
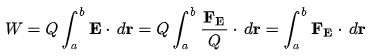
Here, Q = charge on the particle.
E = strength of electric field,
FE = Coulomb force
r = displacement of particle under the action of the Coulomb force.
Magnetic Work:
The work done by unit magnetic field in moving an unit charge is called as magnetic work. It is given by,
W = F dR
Where, F = force of magnetic field = qv X B
dr = distance of movement of charge.
Gravitational Work:
The work done in moving any body against the force of gravity is called as gravitational work.
Force due to gravity = F = mg
Gravitational work = F x h = mgh
Where, h = lift of the particle against the force of gravity.
Spring Work:
When a force is applied on spring, it compresses or expands, depending on the direction of the applied force.
This displacement causes some work to be done on the spring.
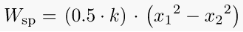
Where, k = stiffness constant of the spring,
x1 = initial distance of the spring from datum
x2 = final distance of the spring from the datum
Shaft Work:
When a shaft is rotated by some external force, work is done on the shaft.
It is given by,
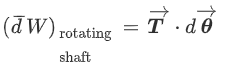
Where, T= torque acting on the shaft
dθ = angular displacement in radians.