Unit 3
Definition
Properties of Pure substances
A substance that has a homogeneously fixed chemical composition is called a pure substance.
In thermodynamics, Steam is considered as a pure substance.
So, in this module, we are going to study about the various processes and properties of steam.
Formation of steam
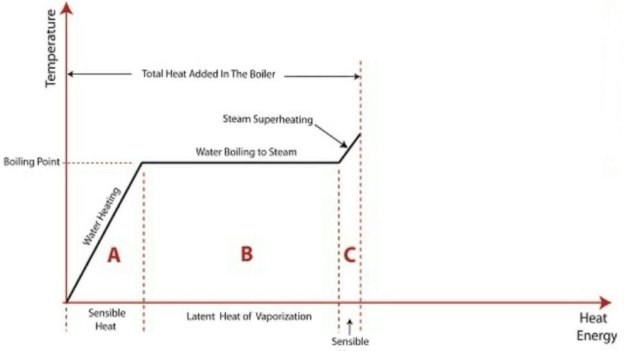
The above diagram shows the phase change occurring when water from and at 0°C is heated till is converted to steam and further to superheat it.
Temperature is plotted as ordinate and Enthalpy as abscissa.
Phase Change
hg = hf + hfg
hsup = hg + Cp (tsup - tsat)
tsup = temperature of superheated steam
tsat = temperature of saturation of steam
Cp = Specific heat of water at constant pressure.
PROPERTY DIAGRAMS FOR PHASE-CHANGE PROCESSES
In order to understand the change in properties of water and steam during the phase change process, we have to study the property diagrams.
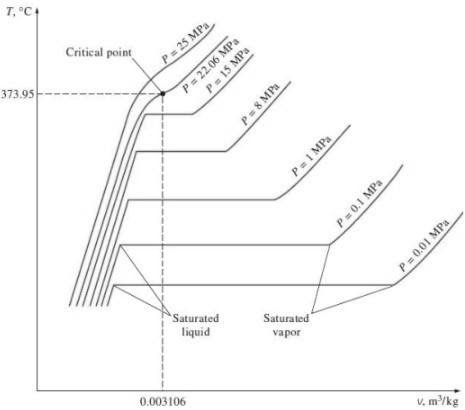
Critical Point
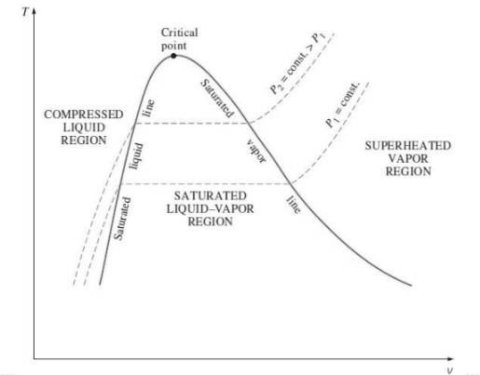
2. P-v diagram for pure substance:
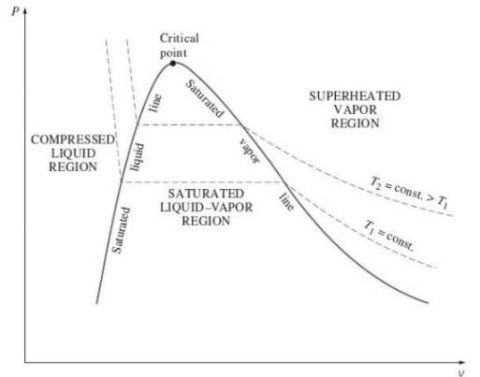
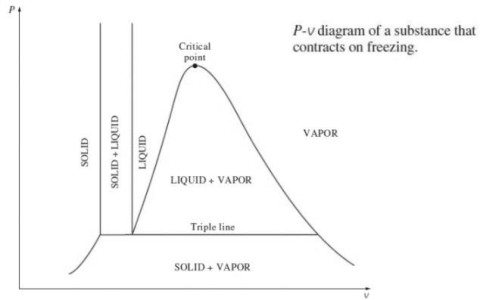
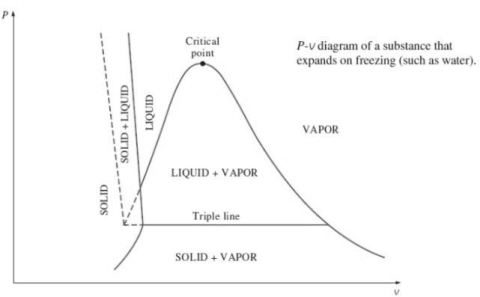
Triple point
3. P-T diagram for pure substance:
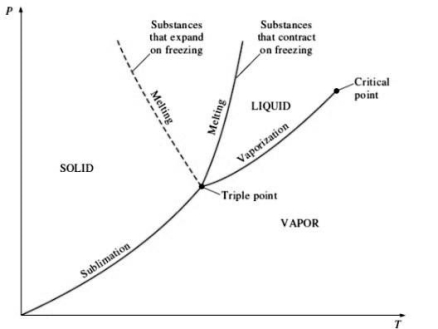
STEAM TABLES & THEIR USES
For a wide range, it is very difficult and complex to keep track of various relationships of thermodynamic properties.
Hence, these are tabulated to make it easier for engineers to find various values.
For water, these are called as STEAM TABLES.
There are 3 notations used.
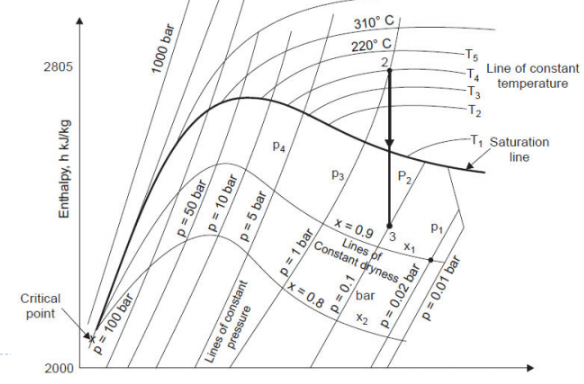
ENTHALPY-ENTROPY (h-s) CHART OR MOLLIER DIAGRAM
It was prepared by Dr. Mollier. It shows relation between enthalpy and entropy or water at various pressures. It is very useful. The steam tables are actually tabulated version of Mollier diagram.