Unit 5
Second law
Second Law of Thermodynamics:
STATEMENTS OF SECOND LAW OF THERMODYNAMICS:
The second law of thermodynamics has two statements put forward by two scientists.
Although the Clausius and Kelvin-Planck statements appear to be different, they are really equivalent in the sense that a violation of either statement implies violation of other.
Equivalence of Clausius Statement to the Kelvin-Planck Statement
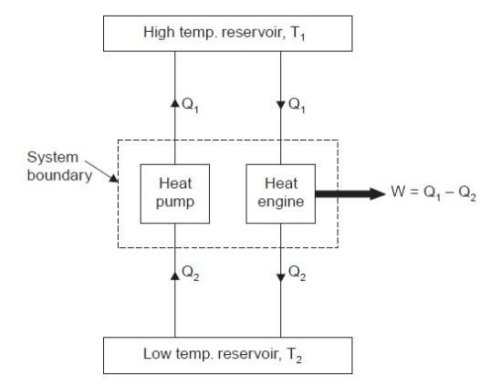
Consider a higher temperature reservoir T1 and low temperature reservoir T2. Fig. shows a heat pump which requires no work and transfers an amount of Q2 from a low temperature to a higher temperature reservoir (which is a violation of the Clausius statement).
Let an amount of heat Q1 (greater than Q2) be transferred from high temperature reservoir to heat engine which develops a net work, W = Q1 – Q2 and rejects Q2 to the low temperature reservoir. Since there is no heat interaction with the low temperature, it can be eliminated. The combined system of the heat engine and heat pump acts then like a heat engine exchanging heat with a single reservoir, which is the violation of the Kelvin-Planck statement.
Heat Engine:
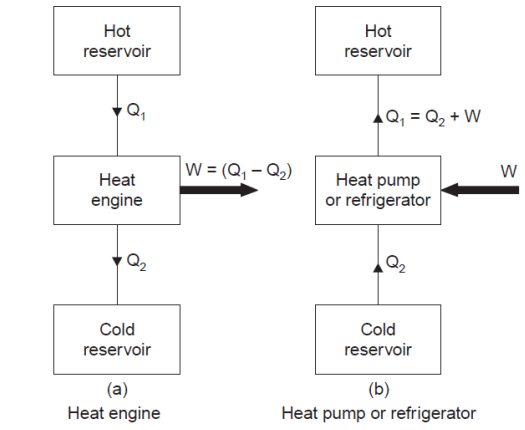
The above figure shows a heat engine and a reversed heat engine which is also called as heat pump.
Heat engine is a machine that can extract heat from a body at higher temperature and convert it into work. The losses are assumed to be dissipated to a body at lower temperature called sink.
A heat engine is used to produce the maximum work transfer from a given positive heat transfer.
The measure of success is called the thermal efficiency of the engine and is defined by the ratio:

where, W = Net work transfer from the engine, and
Q1 = Heat transfer to engine.
For a reversed heat engine acting as a refrigerator when the purpose is to achieve the maximum heat transfer from the cold reservoir, the measure of success is called the co-efficient of performance (C.O.P.). It is defined by the ratio:
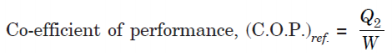
where, Q2 = Heat transfer from cold reservoir, and
W = The net work transfer to the refrigerator.
For a reversed heat engine acting as a heat pump, the measure of success is again called the co-efficient of performance. It is defined by the ratio:
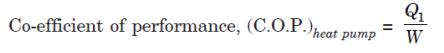
Where, Q1 = Heat transfer to hot reservoir,
REVERSIBLE PROCESSES
A reversible process should fulfil the following conditions:
1. Frictionless process.
2. Heat transfer should not take place with finite temperature difference.
3. The energy transfer as heat and work during the forward process should be identically equal to energy transfer as heat and work during the reversal of the process.
4. There should be no free or unrestricted expansion.
5. There should be no mixing of the fluids.
6. The process must proceed in a series of equilibrium states.
THE CARNOT VAPOR CYCLE
The Carnot cycle is the most efficient cycle operating between two specified temperature limits. Thus, it is natural to look at the Carnot cycle first as a prospective ideal cycle for vapour power plants.
However, the Carnot cycle is not a suitable model for power cycles.
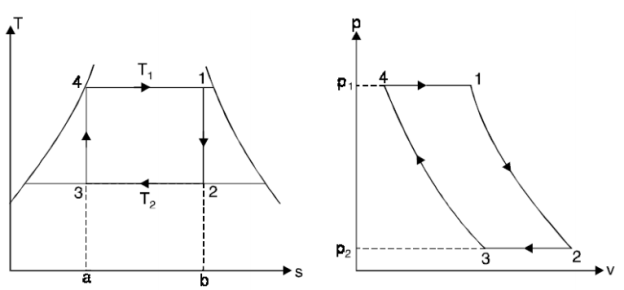
Thus, cycle is completed.
Net work done = Heat supplied – heat rejected
= T1 (s2 - s3) - T2 (s2 - s3)
= (T1 – T2) (s2 - s3)
Carnot Efficiency η = Work done / Heat supplied = (T1 – T2) (s2 - s3) / T1 (s2 - s3)
= (T1 – T2) / T1
Limitations or Impracticalities of Carnot Cycle:
Though Carnot cycle is simple (thermodynamically) and has the highest thermal efficiency for given values of T1 and T2, yet it is extremely difficult to operate in practice because of the following reasons:
Some of these problems could be eliminated by executing the Carnot cycle in a different way, as shown in Fig. B.
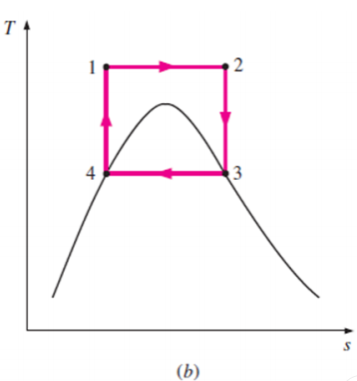
This cycle, however, presents other problems such as isentropic compression to extremely high pressures and isothermal heat transfer at variable pressures.
Thus, we conclude that the Carnot cycle cannot be approximated in actual devices and is not a realistic model for vapor power cycles.