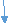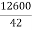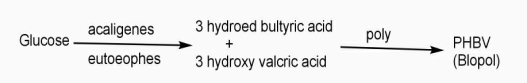UNIT 3
ENGINEERING MATERIALS
Engineering materials: - Engineering materials are the group of materials which are used in the construction of manmade structure and components.
Speciality polymers: - New technological challenges have forced scientist to prepare polymeric materials which are very special in their applications. These are called speciality polymers like self-heatingplastic,clothes, conducting polymers, biodegradable plastic,etc.
Speciality polymers are: -
Which are designed for a specific application or special purpose. Speciality polymers can provide multifunctional properties e.g. Thicker in paint may also act as a dispersant for the pigment.
Characteristics of speciality polymers: -
Examples: - In water treatment, paper processing, textile industry, drug delivery, sensors etc. Basic terms
1) Monomer: - It is a chemical substance it gets convert. Into polymer by polymerisation it has low mol. n+. E.g.– vinyl chloride
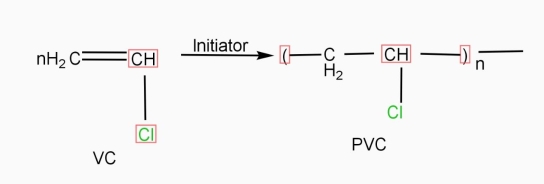
C l 2) Polymer: - It is a chemical substance which obtained by joining large no. of repeating units (monomers) by polymerization. it has high Mol.wt. e.g. – pvc, Nylon6,ps, pp.
3) Polymerization: - It is a chemical process in which monomer get converted into polymer called as polymerization.
Example: -
4) Degree of polymerization: -(DP) It is the average number of monomers present in the molecule of polymer M = n. Mo OR Mw = m. DP
Therefore, M/Mw = mass of polymer M / Mo = mass of monomer N / DP = degree of polymerization
Numerical: -
1) A monomer of mol. wt 42 which form a polymer of average mol. wt 12600, calculate degree of polymerization ( 2 M )
Solution: -
Given: - M = 42 M = 12600
Formula = M = n. Mo 12600 = n.42
Therefore, Degree of polymerization = n =
5) Functionality of monomer: - It is the total number of reacting sites or functional group present in molecule of monomer. Monomer molecule should contain at least two reacting positions / functional groups to convert into polymer.
Types of functionality
1) Bi functional: - If monomer molecule contains two reacting positions / functional group, then it is called as bi functional monomer
R
2) Tri functional monomer: - If monomer molecule contains three reactive positions or functional groups. Then is called as tri functional monomer. It from cross linked polymers e.g.: -
3) Tetra functional monomer: - If monomer molecule contains four reactive position or functional groups. Then it is called as tetra function monomer. E.g. –
Classification of polymers: -
Based on thermal effect behaviour
There are two types
|
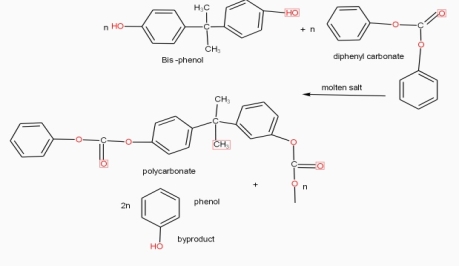
Engineering Thermoplastic: Polycarbonate
|
Properties: -
- PC is high performance tough, amorphous and transparent thermoplastic polymer. Which has an organic functional group linked together by carbonate groups and offer unique combinations of properties.
- High impact strength.
- Very high tensile strength.
- Transparent, RI = 1.58.
- Dissolves in organic solvents.
- Heat and flame resistivity.
- Specific gravity id 1.2 gm / cc.
- TG (glasstransition temp.) = 145°C
- Tm (meltingtemp.) = 230 - 250° C
Applications / Uses: -
- Act as bullet proof material for making windows of vehicles and houses.
- Used as an insulator in electronics.
- It is used for making CD and DV.D.
- For making Handles of screw drivers etc.
- For making helmets; cell phones etc.
Bio-degradable polymers
(polyhydroxy butyrate – hydroxyl Valente)
Biodegradation: -
It is a processes of converting polymer material into harmless simple gaseous products by the action on enzymes of microorganism and water.
A polymer which can be converted to harmless gaseous products by action of biological enzyme and water, is called as biodegradable polymers 3 components are important in the biodegradation of organic polymers.
A) Organisms: - like pseudomonas,bacillus,protozoa,acetobacter, various fungi,etc.
B) Environment: - suitable temp, moist condition, presence of salts, suitable ph.
C) Nature of polymer: -
- Polymer chain should contain bond which can be hydrolysed or oxidized by the enzyme action o, s.
- High aromatic they are tough for degradation
Hydrophilic chain backbone of polymer contains O,N,3. atoms easily degrade.
3. Hydrophilic end groups
4. More amorphous nature of polymer
5. Small size of polymer material
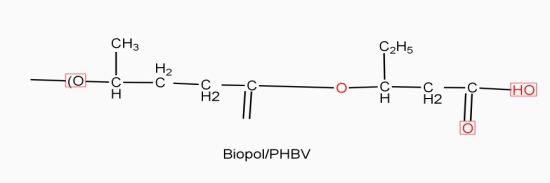
Example: - PHBV
It is a co – polymer of hydroxy butyric acid and 3 hydroxy valeric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.
STRUTURE
|
Properties: -
- It is highly crystalline, soluble in chloroform
- It has melting point 180° c
- It is thermo softening polymer,soft, flexible.
Limitations: -
- Such polymers are very costly
- They cannot manufacture on a large scale
- Don’t possess high mechanical strength
Applications: -
- Used for moulded articles
- Used for films for packing and lamination
- It is used in medical field – for organs transplants,surgical, and orthopaedic, operations.
- It is used in agriculture field – for manufacturing fertilizers and pesticides.
Conducting Polymer
The polymers have ability to conduct electricity are called as conducting polymers.
Structural requirements of polymer: -
- Polymer containing conjugation double bonds system
- Polymer with the higher crystallinity and high planarity
- Presence of aromatic rings in a polymer chain.
- Polymer has a linear structure.
|
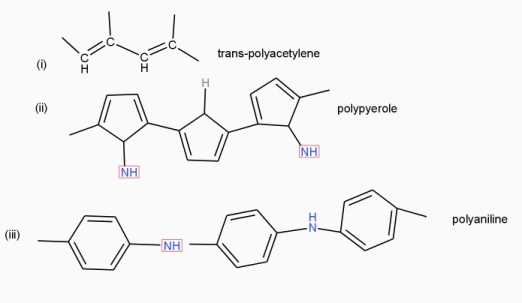
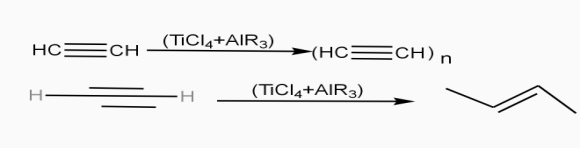
Poly acetylene: -
Structure: -
- The polymer consists of along chain carbon atoms with alternating single and double bonds between them. Each carbon possesses one hydrogen atom.it is obtained by Ziegler matter ayteslys.
|
- Dopling of polymer: - addition of impurities
- Intrinsicpolymers: - the polymer which conduct electricity on their own is called as intrinsic polymers.
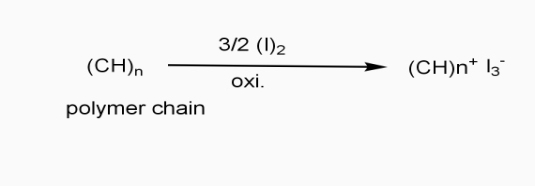
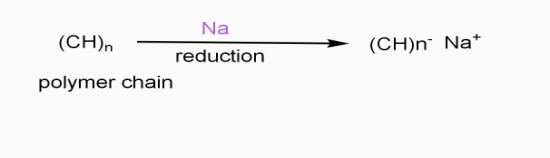
- Extrinsic polymers: - the polymers which conduct electricity by doping processes are called as extinct polymers.
In doping process, polymer is either oxidized or reduced.
There are two type of doping
a] oxidative / p type doping
b] Reductive / n type doping
Applications: -
|
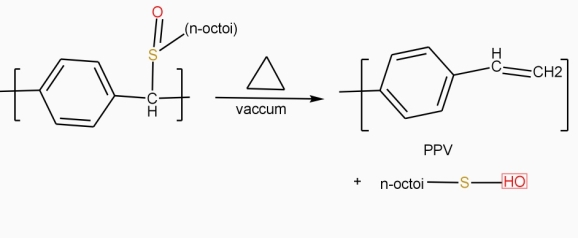
Electroluminescent polymer (PPV): -
‘Property in which material shows / produce bright light of different colours, when stimulated electronically called as electroluminescence property‘.
The polymer which shows electroluminescent property is called as electroluminescent polymer.
e.g. – PPV 9 polyporaphenylenevinylene)
|
Mechanism: -
- Electroluminescent is the result of radioactive recombination of electrons and holes in a material usually a semi – conductor.
- The erected electrons release their energy as photons – light.
Properties: -
- PPV is a diamagnetic material and has very low intrinsic electrical conductivity.
- The electrical conductivity increases upon doping with I2,formicchloride, alkali metals or acidic.
Applications: -
- In photovoltaic cells
- Long life, full colour displays.
- In electroluminescent night lamps.
- Arrow light decoration.
- Back light for liquid crystal displays.
- Theatre assembly hall decoration.
Usually it is used as mixture with (6.6) phenyl 60 – bytyric acid methyl ester (PCBM ) For applications.
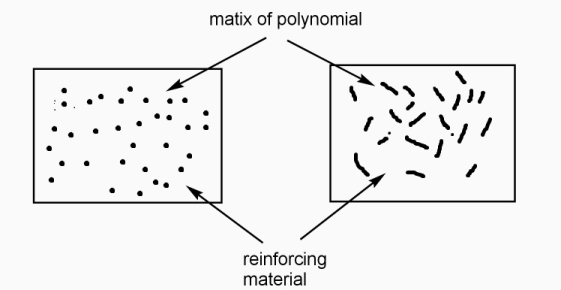
Polymer composite / fibre reinforcing plastics (F R P): -
Definition: -
Polymer and reinforcing materials as a two-phase mixture with the interfaces between them, called as polymer composite.
Therefore,
Polymer phase called as ‘matrix phase ‘mixing constituents called as dispersed phase and boundaries between the matrix and dispersed phase, are known as interface.
|
a) Particle refinement
b) Fibre refinement
Functions of matrix constituents in polymer composite: -
- To bind the reinforcing particles / fibres strongly.
- It acts as medium for distribution of applied load to the dispersed phase.
- It keeps the reinforcing fibres in proper orientation for high strength development.
- It prevents propagation of cracks due to plasticity.
Classification of composites
A] particle reinforcement: - it also called as particulate composites.
- These are composed of particles distributed or embedded in the matrix body. metallic or non-metallic particles can be added to polymer matrix to improve mechanical strength.
- E.g. – carbon black reinforced rubber show improvement in tensile strength,toughness and abrasion resistance and are used in manufacturing automobile tyre.
B) fibre reinforcedcomposites: -
These are composed on fibres embedded in the matrix material. these can be further divided into 3 categories – continuous aligned, discontinuous aligned and discontinuous randomly oriented fibre composites.
e.g. – glass fibre reinforced composite, carbon fibre reinforced and aramid fibre reinforced composites.
Technique: -
- Resin is coated on fibre cloth or fibre mat on both the sides.
- Rollers are used to spread the resin uniformly.
- Alternative layers of the resin – fibre material arranged with same or different orientation for getting proper thickness then passing through mould for proper temperature.
Structural composites: -
(2types)
- Laminar – consists of sheets or panels with proper orientations and cemented together with a resin.
e.g. – plywood, high tensile strength.
2. Sandwiched – consists of two strong outer sheets separated by a layer of less dense material called as core.
Properties of composite: -
- Low coefficient of expansion
- Low cost of production
- High dimensional stability
- High tensile strength
- High heat stability, higher working temp.
- Better abrasion / wear resistivity.
- Better toughness and impact strength.
Applications: -
- In automobile parts, racing vehicles components.
- Parts of aircrafts.
- Boats parts.
- Sport goods, musical instruments, various toys,etc.
- High speed machinery parts,PCBS, equipment parts, bodies of refrigerator,coolers, windows and doors,etc.
Nano materials: -
Nano technology: -
It is defined as the study and use of particles between 1 nanometre and 100 nano meters in size.
Nano material: -
When at least one of the dimensions of particles of material is in the range of 1 to 100 nm then such a material is called as nano material.
(1nm = 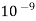 )
)
classification of nanomaterials based on dimensions (zerodimensional, one-dimensional, two-dimensional and three-dimensional),
Based on dimension, there are 4 types of nano material.
A] zero-dimensional nano material: -
The nano materials have all dimensions within nanoscale range and larger than 100 nm are the zero-dimensional(OD) nanomaterial.
e.g. – quantum dots,particles,heterogeneousparticles.
B] one dimensional nano material: -
These are the nanomaterial having grown along one dimension beyond nano scales.
e.g. – nano wires, nano rods.
C] the dimensional nano material: -
Two-dimensional nanomaterial has two dimensions outside the nano scale.
e.g.: - carbon nano tubes, nano plates, nano sheets.
D] three-dimensional nano material: -
Three-dimensional nanomaterial is the bulk nanomaterial which are not confined to nanoscale in any dimension.
e.g.: -fullerenes, bundles of nanotubes,etc.
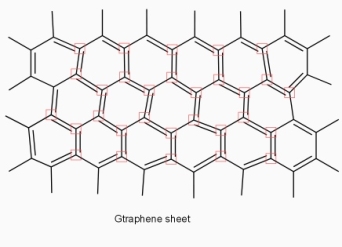
graphene: -
C =  ,
, 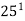 ,
, 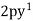 .
.
Structure
- All the carbon atoms in grapheme are sp2 hybridised.
- Graphene – it is a single layer of carbon atoms organized in hexagonal lattice.
- Two-dimensional grapheme molecules sp2 carbon has three hybrid orbitals at 120 degrees angle in one plane and one unhybride orbital. having one electron at right angle to the plane.
- Hexagonal arranged networks sheet of carbon atoms is formed by joining of each sp2 carbon to 3 other sp2 carbon by 6 bonds. This way of joining the carbons, result in forming huge and flat molecules with millions of carbon atoms in the plane.
- Bond length – 1.42 A degree
- Bond angle – 120 degree
|
Properties
- C – C bond length is 0.142 nm
- It has electrical, thermal and physical properties.
- High electrical conductivity
- High opacity and strength
- It is thinnest but strongest material
In detail
- Electronic properties: -
It is one of the best electrical conductors on earth. the unique atomic arrangement of carbon atoms in grapheme allows its electrons to easily travel at extremely high velocity.
2. Stiffness: -
The breaking force obtained experiment and from simulation is almost identical and the experimental value of the second order elastic stiffness was equal to 380 nm
3. Strength: -
Grapheme is considered as a strongest material with a strength 42 nm.
4. Toughness: -
Fracture toughness, which is a property very relevant to engineering applications is one of the most important mechanical properties of grapheme and was measured as a critical stress.
5. Mechanical properties: -
The impressive intrinsic mechanical properties of grapheme its stiffness strength and toughness are one of the reasons that make grapheme stand out both as an individual material as a reinforcing agent in composites.
6. In chemical reaction: -
It is the only form of carbon in which every atom is available for chemical reaction from two sides (due to 2D structure)
Graphene
Preparation of graphene: -
- Graphene synthesis – chemical vapour deposition (CVD) is an effective way / method to produce high quality graphene on a large scale.
- Sodium metal is slowly heated with ethanol for 3 days and then rapidly heated the resulting solid consists of a fused graphene sheets which are then washed and dried, but quality of sheets is not so good by this method.
- Graphite surface is exfoliated with adhesive to give thin flask of graphene.
Application / uses
- As a sensor for gas detection
- In transistors
- In batteries
- For gas storage
- In making transparent conducting electrodes
- In solar cells
Fullerenes / Buckyball: -
- It is one of the allotropes of carbon it was discovered during the experiment.i.e. laser vaporization of graphite rod under high vacuum chamber by scientist ‘kroto ‘.
- Fullerenes are cage like carbon cluster having formula c60, c70, and c78.
- C60 fullerene is stable and spherical.
- All carbon atoms in fullerenes are on the surface of sphere or ball therefore it is called as Bucky ball.
Structure: -
- In c60 fullerene 12 pentagons and 20 hexagons of C atoms are present with restriction not two pentagon touch to each other.
Diameter is 0.7 nm to 7 a
- Total C = C are 30
- Structure in a such way that possible to trap some ions.
- Has a structure as fcc
- It is a semiconductor material
- Density 1.65 gm / cceach C in fullerene is covalently bonded with 3 other such carbon atoms.
- Electronic configuration
 =
= 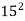 ,
, 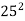 ,
, 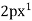 ,
, 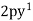 ,
, 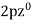
Es = 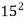 ,
, 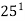 ,
, 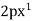 ,
,  ,
, 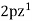
- At higher temperature graphite temperature graphite rod break down to form a spherical fullerene.
Properties: -
- All ‘c‘atoms are equivalent in C60 fullerene
- It shows poor automaticstructure.
- It shows more character like alkanes.
- It acts as an electron acceptor.
- It can undergo photonics association processes
Applications / uses: -
- It is a semiconductor but from super conductor with alkali metals.
- C60 participate in catalytic process.
- In optical devices
- For trapping of small particles.
- In medical field used as diagnostic and therapeutic agent. Inhibitors to HIV
Carbon nanotubes (CNTS): -
It is a two-dimensional nanomaterial CNT can be imagined to be formed by folding of a graphite sheet just like a rolling of paper into a cylinder form CNTs can be considered as the cylinders made up of graphite sheets.
There are two type of CNT
A] single walled carbon nanotubes (SWCNTS): -
It is the single folding of thick layer of graphite sheet.
B] multi walled carbon nanotubes (MWCNTS) or concentric CNT: - It is a multiple rolled layer of graphite
3 types of SWCNT
It is achiral l and semi conduct material. II. Armchair: - It is achiral. it shows electrical conductivity.
III. Helical: -
It is chiral also act as semiconductor.
Synthesis of carbon nanotubes: - There are two methods of synthesis of CNTs A] electric isdischarge: - An electric arc is struck between two graphite electrodes under following conditions, to manufacture the mixture of SWCNT andMWCNT.
Conditions: -
Processes: -
Length of CNT = few micrometre
B] chemical vapour deposition: - This method used on a large scale
|
Quantum Dots
DEFINITION: -
1] quantum dots: -
Quantum dots are semiconductor nanoparticles that glow a colour after being illuminated by light.
Quantum dots are zero dimensional nanomaterials. they are fluorescent nanoparticles that are invisible until it up by uv light.
2] definition: -
Quantum dots are nanoparticles that exhibits three-dimensional confinement which leads to many unique optical and transport properties.
They can exhibit a range of colours depending upon their composition and size.
Properties of quantum dots: -
1] optoelectronic properties: -
Quantum dots have properties intermediate between bulk semiconductor and discrete atoms or molecules.
The properties of a quantum dot are determined by size,shape, composition and structure. their optoelectronic properties change as a function of both size and shape. Many semiconductor substances can be used as quantum dots.
2] fluorescence: -
In semiconductor, light absorption generally leads to an electron being excited from the valence to the conduction band, leaving behind a hole.
The electron and the hole can bind to each other to form an excitation‘s energy can be emitted as light. this called as fluorescence as the confinement energy depends upon the quantum dots size, both absorption and fluorescence emission can be tuned by changing the size of the quantum dot during its synthesis.
Applications of quantum dots: -
1] electronics: -
Quantum dots have various applications in several areas such as solar cells,transistors,LEDS, medical imaging and quantum computing because of their unique electronic properties.
2] photocatalysts: -
Quantum dots also function as photocatalyst for the light driven chemical conversion of water into hydrogen as a pathway to solar fuel.
3] in biology: -
Quantum dots have antibacterial properties similar to nanoparticles and can kill bacteria.
It is used for tumour targeting under in vivo conditions.
4] light emitting diodes: -
Quantum dot light emitting diodes (QD – LED) and qd – white led. are very useful when producing the displays for electronic devices. QD – LED displays can give colours very accurately and use much less.
References: -
1. Engineering Chemistry by O.G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr. Sunita Rattan, S. K. Kataria& Sons Publisher